High Dose Vitamin C Therapy for Dapsone Induced Methemoglobinemia
Sabrina Genovese1,*, Brooklyn Campbell1, Robert Lavery2, Preethi Yerram3, Shreepriya Mangalgi3 and Andrew Harding4
1University of Missouri, School of Medicine, Columbia MO, USA
2University of Missouri, Department of Internal Medicine, Columbia MO, USA
3University of Missouri, Department of Nephrology, Columbia MO, USA
4Harry S. Truman Memorial Veterans' Hospital, Department of Internal Medicine, Columbia MO, USA
Received Date: 22/12/2024; Published Date: 17/02/2025
*Corresponding author: Sabrina Genovese, BS, University of Missouri, School of Medicine, 1 Hospital Dr, Columbia, MO 65201, USA
Abstract
Methemoglobinemia is a potentially fatal condition in where hemoglobin is oxidized to its ferric state, reducing oxygen-carrying capacity and causing tissue hypoxia. It can be treated with methylene blue (MB), though it is contraindicated in Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency due to risk of hemolysis. This case describes an 86-year-old male with membranous nephropathy, chronic kidney disease and unknown G6PD status who developed methemoglobinemia after receiving dapsone for Pneumocystis jirovecii pneumonia prophylaxis. He exhibited worsening hypoxia, altered mental status, and chocolate-colored blood. After MB administration, he experienced an acute hemoglobin drop, treatment was transitioned to high-dose vitamin C resulting in clinical improvement. This case supports vitamin C as a safe alternative therapy when MB is contraindicated. It emphasizes the importance of clinical judgment in diagnosing and treating conditions like methemoglobinemia, where pulse oximetry may provide misleading results. Further research is needed to establish guidelines for vitamin C in methemoglobinemia treatment.
Keywords: Methemoglobinemia; Vitamin C; Ascorbic Acid; Dapsone; Chronic Kidney Disease; G6PD
Introduction
Methemoglobinemia is cause of hypoxia in which iron within hemoglobin is oxidized from a ferrous state (Fe2+) to a ferric state (Fe3+). Ferric iron irreversibly binds oxygen, leading to reduced oxygen-carrying capacity, impaired oxygen unloading, and subsequent tissue ischemia [1,2]. Methemoglobinemia can be due to an autosomal recessive defect in nicotinamide adenine dinucleotide (NADH) cytochrome b5 reductase, but much more commonly due to drugs or toxins [3,4]. Dapsone, local anesthetics, and antimalarial drugs are known oxidizing agents that most frequently cause methemoglobinemia [3]. Clinically, methemoglobinemia classically presents as dyspnea and hypoxemia refractory to supplemental oxygen [5]. It may also present with headache, fatigue, dizziness, metabolic acidosis and in severe cases coma and death [5,6] Traditional dual-wavelength pulse oximetry provide unreliable measurements of oxygen saturation as methemoglobin absorbs light at both 660 and 940 nm, interfering with the measurement. When methemoglobin levels reach 30-35%, the absorbance ratio becomes 1.0, which falsely reads as an SpO2 of 85% [5]. The gold standard therapy has been methylene blue (MB) which acts as an oxidizing agent, accepting an electron from nicotinamide adenine dinucleotide phosphate (NADPH) for reduction of ferric to ferrous iron [2,5]. However, methylene blue therapy is contraindicated in patients with Glucose-6-phosphate dehydrogenase (G6PD) deficiencies as high doses of leucomethylene blue, a reducing agent byproduct, cause life-threatening intravascular hemolysis [7] Additionally, certain countries such as Korea have MB shortages due to import suspensions [8]. Thus, it is important to identify other safe and effective therapies for methemoglobinemia. We present a case of dapsone induced methemoglobinemia treated with high dose vitamin C therapy.
Case Presentation
An 86-year-old man admitted to Harry S. Truman Memorial Veterans' Hospital for fatigue and worsening bilateral edema of the lower extremities and a reported 20lb weight gain. He had stage III chronic kidney disease, membranous nephropathy, hypertension, atrial-fibrillation, post-traumatic stress disorder and obstructive sleep apnea. Physical examination revealed 3+ pitting edema from bilateral knees to the feet and 2+ pitting edema in bilateral thighs. Labs showed hyponatremia (122mmol/L), hyperkalemia (5.3mmol/L) and elevated creatinine (2.69mg/dL), with a baseline creatinine of 2.15mg/dL. He was previously treated with Rituximab but discontinued due to serum sickness. He was transitioned to 100mg cyclosporine twice daily which he had been tolerating well. After workup, he was diagnosed with relapsing membranous nephropathy. Cyclosporine was discontinued due to rising creatinine levels, and he was transitioned to intravenous (IV) Furosemide 80mg daily.
Nephology was consulted for assistance in management relapsing membranous nephropathy. Per nephrology recommendations, the patient received 3 doses of IV methylprednisone 500mg daily, mycophenolate mofetil 500mg twice daily, prednisone 60mg once daily to treat his relapsing membranous nephropathy. He was also placed on a fluid and salt restricted diet. After 10 days of steroid therapy, he was started on Pneumocystis jirovecii pneumonia prophylaxis. Given poor renal function, dapsone 100mg daily was chosen over first line therapy Trimethoprim/sulfamethoxazole therapy for concerns of renal toxicity. Four days later, he began to exhibit signs of hypoxia, asterixis and alerted mental status. His oxygen requirement began to progressively increase, escalating from baseline room air to high flow nasal cannula. However, SpO2 continued to range from 90-96%. He was transferred to a stepdown unit, where he underwent a full pulmonary and infectious workup.
Initial workup including complete blood count (CBC), comprehensive metabolic panel (CMP) and portable AP chest x-ray, showed no signs of infection but revealed elevated lactic acid (2.8mmol/L) and decreased hemoglobin (9mg/dL). Arterial blood gas (ABGs) showed a concerning mixed respiratory and metabolic alkalosis (pH 7.76, pCO2 21, HCO3 34.3). Patient was started on Vapotherm high flow nasal cannula, 25L and 100% FiO2. His status continued to rapidly decline, with worsening mental status and weakness. Pulmonology was consulted and identified no obvious pulmonary cause of hypoxia. During routine labs, a nurse alerted the team about "chocolate-colored" blood discoloration. A methemoglobin level was drawn and was critically high at 22.1%. 75mg of IV MB was administered over 30 minutes. Subsequently, an acute hemoglobin drop from 8mg/dL to 7mg/dL was noted requiring 1 unit packed red blood cell transfusion and prompting concern for hemolysis in a patient with unknown G6PD status. He was transitioned to high dose vitamin C therapy (5000mg, IV once) and monitored for clinical improvement. He remained encephalopathic with repeat ABGs showing continued alkalosis (pH 7.84, pCO2 16.5, PO2 of 244). Vapotherm was discontinued, and the patient was switched to 7L O2 via nasal cannula, with SaO2 in the mid 80s. Two additional doses of high dose oral Vitamin C (5000mg, oral) were administered. Repeat methemoglobin levels were 10.6%. His clinical picture slowly improved.
Hematology-Oncology was consulted for concerns intravascular hemolysis, who believed the anemia to be multifactorial -- a combination of dapsone-induced methemoglobinemia and MB administration. He was restarted on prednisone 20mg daily and mycophenolate mofetil 250mg twice daily as he continued to improve. He was restarted on Pneumocystis jirovecii pneumonia prophylaxis with sulfamethoxazole 400mg/trimethoprim 80mg renally dosed.
The patient remained in the hospital for an additional 25 days receiving close laboratory monitoring and physical and occupational therapy 4-6 times per week. He was discharged to a skilled rehab facility due to weakness from a prolonged hospital stay. His labs at discharge were as follows: hemoglobin 10.5g/dL, BUN 37 mg/dL and creatinine 1.71 mg/dL. He was advised to continue prednisone 20mg daily for 2 weeks, then 10mg daily for 1 month in addition to mycophenolate mofetil 250mg twice daily until follow up with nephrology outpatient.
Discussion
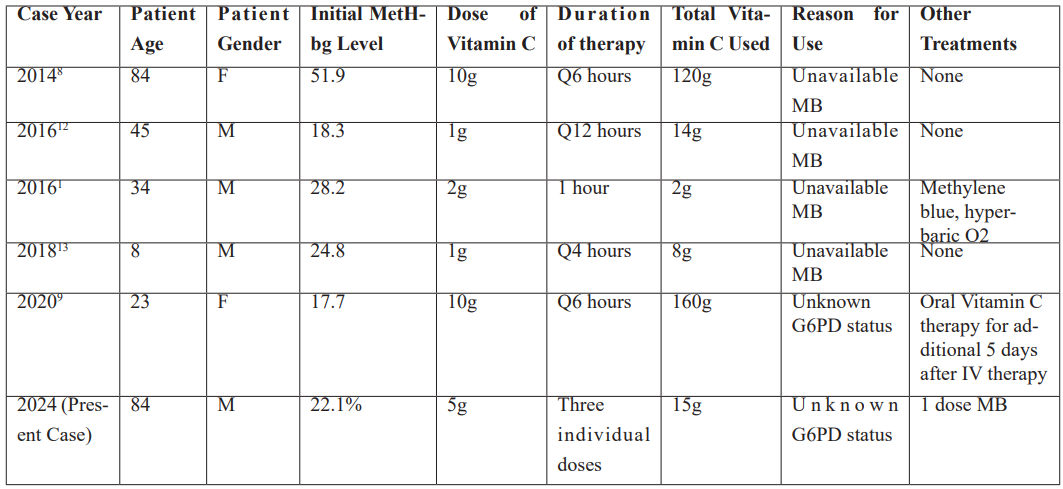
Existing literature demonstrates 5 cases (Table 1) where patients with varying levels of dapsone induced methemoglobinemia were treated with vitamin C. The cases vary by patient age, gender, and treatment regimen but highlights that vitamin C was used in various doses and frequencies, with some cases requiring additional treatments such as MB or hyperbaric oxygen. In 2016, a 45-year-old patient with an initial methemoglobin level of 18.3% received 1g of vitamin C every 12 hours, totaling 14g, without any other treatments. The same year, a 34-year-old patient with an initial methemoglobin level of 28.2% was treated with a single 2g dose, along with MB and hyperbaric oxygen. These case reports reveal vitamin C to be an effective alternative treatment for methemoglobinemia. The consistent success across varying levels of Methemoglobinemia suggests that vitamin C is a safe option for individuals ranging from 8 to 84 years of age.
Dapsone is a sulfonate antibiotic with anti-inflammatory properties [9] used in this case for Pneumocystis jirovecii pneumonia prophylaxis. Dapsone accounts for 42% of all cases of methemoglobinemia [10], due to the metabolites of dapsone which oxidize iron from ferrous to ferric state of hemoglobin forming methemoglobin [9]. It is responsible for continual oxidative stress due to the long half-life (30 or more hours) of the drug [9]. Typically, MB is the first-line treatment in patients with methemoglobin levels exceeding 30%, or 20% when symptomatic [5]. MB relies on the enzyme G6PD to function as an electron donor in reducing methemoglobin. However, in G6PD deficient individuals, hemolysis can precipitate. In this case, after administering MB, the patient experienced a significant drop in hemoglobin levels raising concerns for hemolysis. MB treatment was immediately stopped and transitioned to high-dose vitamin C.
Unlike MB, vitamin C does not utilize the G6PD enzyme but acts as a reducing agent, converting methemoglobin back to hemoglobin via a different biochemical pathway. Vitamin C effectiveness can vary based on dose and patient factors, and it has been most notably studied in its IV form. The pharmacokinetics of vitamin C demonstrate that IV administration results in higher plasma concentrations compared to the oral route, as shown by Padayatty [11]. In their study, a dose-dependent manner increase in plasma vitamin C levels was observed with IV administration, while a plateau effect was observed as oral doses increased. This difference may suggest a limitation of oral treatment, which may not provide the same therapeutic relief as IV, particularly in severe cases of methemoglobinemia. While oral vitamin C is widely and readily accessible, we were compelled to use it due to the depletion of IV vitamin C supplies at this hospital and nearby facilities. Additionally, vitamin C can increase the urinary excretion of oxalate, posing a risk of hyperoxaluria, especially in patients with renal dysfunction. This was not seen in this case. Further studies are required to establish clear guidelines for methemoglobinemia management with Vitamin C.
This case also emphasizes the importance of clinical judgment over relying on medical technology. Despite the patient showing clear signs of respiratory distress, including increased oxygen requirements, asterixis, and altered mental status, his pulse oximeter readings remained in the normal range. Relying solely on pulse oximeter readings could have delayed diagnosis and treatment. Clinicians should prioritize the clinical picture over device readings for timely interventions.
Table 1: Previous cases of Dapsone-induced methemoglobinemia treated with high dose vitamin C therapy.
Conclusion
This case highlights the effective use of vitamin C as a safe alternative treatment for methemoglobinemia, especially in patients with unknown G6PD status. It showcases the importance of relying on clinical judgement over medical devices. It emphasizes the value of multidisciplinary medical management, careful monitoring of underlying comorbidities, adverse drug reactions and the need for individualized care throughout a patient’s entire hospital stay.
Author Contributions: SG and BC drafted the manuscript, RL, SM, PY and AH revised and provided important intellect of the manuscript.
Competing Interest: None to disclose.
Grant Information: The authors received no funding for this work.
References
- Toker I, Yesilaras M, Tur FC, Toktas R. Methemoglobinemia caused by dapsone overdose: Which treatment is best? Turkish Journal of Emergency Medicine, 2015; 15(4): 182-184. doi: 10.1016/j.tjem.2014.09.002.
- Iolascon A, Bianchi P, Andolfo I, et al. Recommendations for diagnosis and treatment of methemoglobinemia. Am J Hematol, 2021; 96(12): 1666-1678. doi: 10.1002/ajh.26340.
- Rehman HU. Methemoglobinemia. West J Med, 2001; 175(3): 193-196. doi: 10.1136/ewjm.175.3.193.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med, 1999; 34(5): 646-656. doi: 10.1016/s0196-0644(99)70167-8.
- Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. In: StatPearls. StatPearls Publishing; 2024.
- Lata K, Janardhanan R. Methemoglobinemia: A Diagnosis Not to Be Missed. The American Journal of Medicine, 2015; 128(10): e45-e46. doi: 10.1016/j.amjmed.2015.04.031.
- Ginimuge PR, Jyothi SD. Methylene blue: revisited. J Anaesthesiol Clin Pharmacol, 2010; 26(4): 517-520.
- Park SY, Lee KW, Kang TS. High-dose vitamin C management in dapsone-induced methemoglobinemia. The American Journal of Emergency Medicine, 2014; 32(6): 684.e1-684.e3. doi: 10.1016/j.ajem.2013.11.036.
- Kabir H, Lakshmanan R, Gopinath S, Bhonagiri D. Dapsone‐induced methemoglobinemia—A case report. Clinical Case Reports, 2021; 9(5): e04054. doi: 10.1002/ccr3.4054.
- Ash-Bernal R, Wise R, Wright SM. Acquired Methemoglobinemia: A Retrospective Series of 138 Cases at 2 Teaching Hospitals. Medicine, 2004; 83(5): 265-273. doi: 10.1097/01.md.0000141096.00377.3f.
- Padayatty SJ, Sun H, Wang Y, et al. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann Intern Med, 2004; 140(7): 533. doi: 10.7326/0003-4819-140-7-200404060-00010.
- Sahu KK, Dhibar DP, Gautam A, Kumar Y, Varma SC. Role of ascorbic acid in the treatment of methemoglobinemia. Turkish Journal of Emergency Medicine, 2016; 16(3): 119-120. doi: 10.1016/j.tjem.2016.07.003.
- Abdelkader SI, Mohmoud AE. Effective role of ascorbic acid as an alternative treatment of methemoglobinemia: A case report. Int J Case Rep Images, 2018; 9: 1. doi: 10.5348/100941Z01EM2018CR.

