Postpartum Cardiomyopathy Masquerading as Asthma Exacerbation: A Critical Case Report
Rachel Andrew1, Precious Idorenyin Anthony2, Navin Patil3,*, Shanti Gurung4, Delia Graham-Durand5, William O Olotu6, Jill Koech6, Favour Enoch Abidoye6, Noodee Al Khinalie6, Karim Slim6, Ram Bhutani7 and Oyindamola Obadare2
1Associate Clinical Dean, All Saints University School of Medicine, Dominica
2Teaching Assistant, All Saints University School of Medicine, Dominica
3Professor and Dean of Basic Sciences, All Saints University School of Medicine, Dominica
4Associate professor and Course Director, All Saints University School of Medicine, Dominica
5Medical Doctor, Dominica China Friendship Hospital, Dominica
6MD student, All Saints University School of Medicine, Dominica
7MD, Internal Medicine, Sinai Hospital of Baltimore, USA
Received Date: 12/12/2024; Published Date: 14/01/2025
*Corresponding author: Dr. Navin Patil, Professor and dean of Basic Sciences, All Saints University School of Medicine, Dominica
Abstract
Background: Postpartum cardiomyopathy (PPCM) presents a significant diagnostic challenge, particularly in women with pre-existing respiratory conditions like asthma, due to overlapping symptoms. This case highlights the importance of considering cardiac causes in postpartum women presenting with respiratory distress.
Case Presentation: A 27-year-old woman, postpartum following an uncomplicated spontaneous vaginal delivery at 39+6 weeks in October 2022, presented with severe respiratory distress and hypotension several months later. With a history of bronchial asthma, her symptoms were initially treated as an asthma exacerbation but persisted despite therapy. Hospital admission prompted further evaluation, excluding pulmonary embolism. Echocardiography subsequently confirmed a diagnosis of PPCM.
Conclusion: This case emphasizes the critical need to distinguish PPCM from asthma exacerbations in postpartum women. Early cardiology consultation and echocardiographic assessment are vital for accurate diagnosis and timely management to avoid severe complications. Increased awareness of PPCM, particularly in patients with pre-existing respiratory conditions, is essential to prevent misdiagnosis. Moreover, understanding the potential worsening of heart failure symptoms with beta-agonists is crucial for effective management and improved patient outcomes.
Keywords: Postpartum cardiomyopathy; Respiratory distress; Beta Agonists
Introduction
Peripartum Cardiomyopathy (PPCM) can be defined as a systolic heart failure that occurs in the final month of pregnancy or within 5 months postpartum, that cannot be attributed to any other underlying cause of heart failure. The ejection fraction in PPCM is defined as <45%. The window of occurrence may be variable in some cases allowing the modification of the definition to include incidences during late pregnancy and the early months post- delivery [1].
Postpartum cardiomyopathy (PPCM) has a variable global incidence, estimated at 1 in 2,000 births. Higher rates are reported in Nigeria (1:100) and rural Haiti (1:300), while Japan records a much lower rate of 1:20,000 births. Race is a significant risk factor, with studies in the United States showing the highest incidence among African American women [2]. Other risk factors include age, a higher risk observed among older women, multiple gestations, Hypertension, and Pre-Eclampsia [3]. The higher incidence rates in developing nations may also highlight possible socioeconomic risk factors associated with PPCM.
The exact cause of PPCM remains unclear but is linked to vasculo-hormonal and inflammatory processes. Insufficient angiogenesis is a proposed mechanism, resulting in myocyte injury and heart dysfunction. Elevated levels of anti-angiogenic factors, including sFlt-1, STAT3, and the 16-kDa prolactin fragment, have been implicated [3].
Two-year mortality rates for PPCM vary significantly, ranging from 0–16% in the U.S. to 15% in Haiti and 28% in Africa, highlighting disparities in healthcare access and management. Early diagnosis and treatment are crucial to reducing mortality [1].
Case
A 27-year-old Afro-Caribbean woman with a history of bronchial asthma presented to the emergency room three months postpartum with chest tightness, weakness, vomiting, and diarrhea (3 episodes of vomiting and 4 of diarrhea in 24 hours). Initially diagnosed with acute gastritis, she received IV fluids, Gravol, Buscopan, and Omeprazole and was discharged with an H. pylori test order.
Obstetric history included G3P0A2 with one uncomplicated vaginal delivery at 39+6 weeks. One month postpartum, she developed progressive shortness of breath, self-treated with a Salbutamol inhaler, later diagnosed as asthma exacerbation by her primary care provider and treated with steroids and continued Salbutamol.
She returned to the ER with worsening symptoms. Vital signs showed BP 99/72 mmHg, pulse 102 bpm, O2 saturation 82–90% on room air, and RR 40 breaths/min. Differential diagnoses included pulmonary embolism and congestive heart failure. ECG findings revealed sinus tachycardia (HR 136 bpm), normal axis, PR interval 120 ms, QRS 40 ms, QT 320 ms, and inverted T waves in V3–V6. Her ECG findings are displayed in Figure 1.
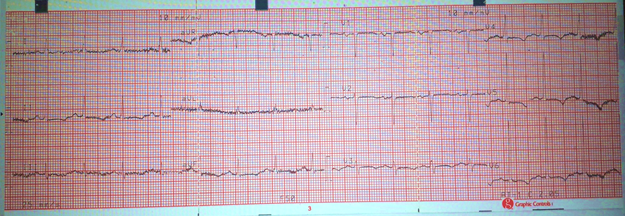
Figure 1: A 12 lead ECG performed on the patient at rest to persistent shortness of breath. Findings include Sinus tachycardia, Inverted T waves in V3-V6.
Investigations showed notable lab results: Troponin <0.02 ng/ml and D-dimer 0.631 µg/ml.
A chest Xray revealed the findings in Figure 2:
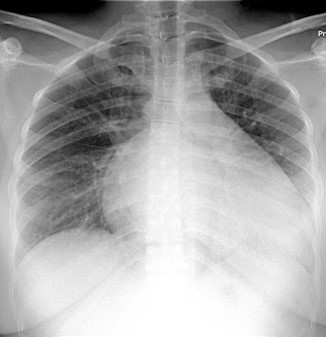
Figure 2: An upright Chest X Ray of a 27-year-old female, showing Increased heart shadow, and patchy opacities in both lungs.
Chest CT with IV contrast ruled out pulmonary embolism. The patient was discharged with Amoxicillin 500 mg (TID for 3 days), Propranolol 20 mg (BID for 1 month), a multivitamin (1 daily for 1 month), an order for an outpatient echocardiogram, and a cardiology referral.
During her final ER visit, she was admitted to the ICU with vitals: BP 84/62 mmHg, pulse 114 bpm, O2 saturation 85–90% on nasal cannula, and RR 44 breaths/min. Exam findings included decreased bilateral air entry, crackles in half of both lung fields, a Grade III/IV mitral murmur, peripheral cyanosis, and +3 bilateral pitting edema.
Significant labs: Troponin 0.17 ng/ml, D-dimer 0.735 µg/ml, PT 16.2 sec, K+ 6 mmol/L, CK-MB 57 U/L. Differential diagnoses included congestive heart failure and mitral valvulopathy. Management involved oxygen, cardiac monitoring, IV Lasix, SQ Lovenox, Dobutamine infusion, and cardiology consultation.
Echocardiogram findings: ejection fraction of 20%, left ventricular akinesis, severe mitral and tricuspid regurgitation, no pulmonary hypertension, and ischemic changes at rest (Figure 3).
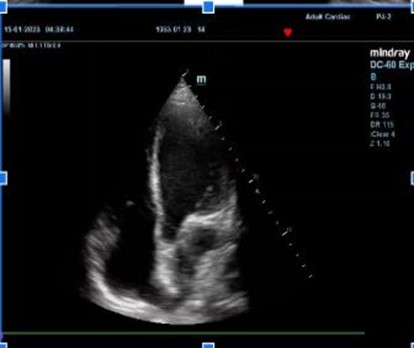
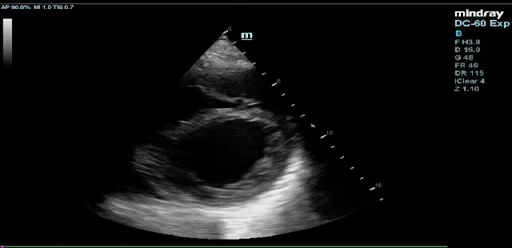
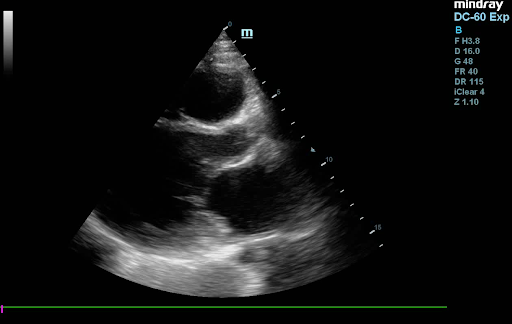
Figure 3: Echocardiogram images displaying in respective order: Apical 4-Chamber view, Parasternal Short Axis view, and Parasternal Long axis View, showing an Ejection Fraction of <20%.
A cardiac MRI without contrast confirmed dilated cardiomyopathy (likely non-ischemic), prominent left ventricular trabeculae, mitral regurgitation, and a reduced ejection fraction of <20%. The final diagnosis was peripartum cardiomyopathy with congestive heart failure.
Management included IV Digoxin (0.5 mg for 1 week) and oral Dapagliflozin (10 mg daily). The patient stayed in the ICU for 5 days before transfer to the medical ward, where she remained for 12 weeks before discharge.
Discharge medications: Furosemide 20 mg BID, Spironolactone 12.5 mg daily, Carvedilol 6.25 mg BID, Sacubitril/Valsartan (24/26) daily, Dapagliflozin 5 mg daily, Digoxin 0.25 mg daily, and Ferrous Sulphate 200 mg BID. Follow-ups with Cardiology and Gastroenterology were scheduled.
At follow-up, her echocardiogram two years post-discharge showed improvement, with an ejection fraction of 35–45% (Figure 4).
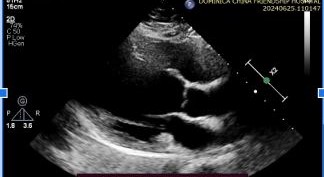
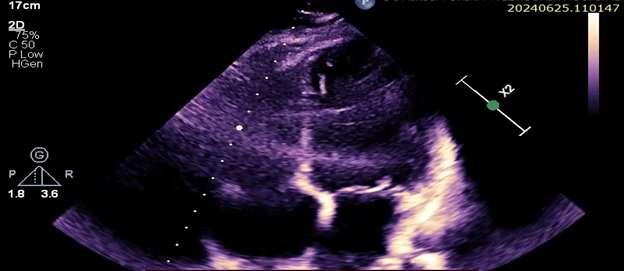
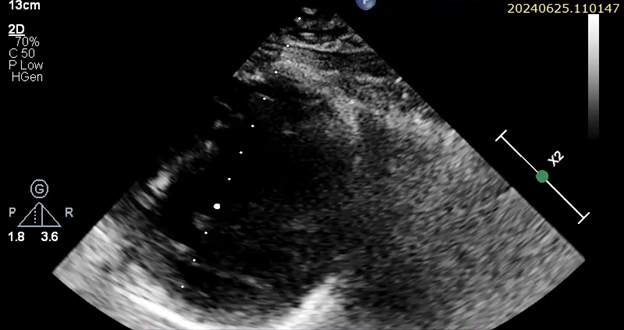
Figure 4: Echocardiogram images displaying in respective order:Parasternal Long Axis, 4-Chamber Apical view, Parasternal short Axis with an Ejection fraction of 35-45%.
Discussion
PPCM is a diagnosis of exclusion, which can lead to misdiagnosis or delays. Complications include brain injury, pulmonary edema, thromboembolic events, and cardiac arrest. Early diagnosis and management can reduce the risk of these adverse outcomes [1]. PPCM presents with symptoms of cardiovascular pathology including dyspnea, orthopnea, peripheral edema, chest pain, cough, and fatigue3. Diagnosing PPCM is challenging due to symptom overlap with other peripartum conditions, such as normal pregnancy changes, pulmonary embolism, amniotic fluid embolism, and myocardial infarction [4]. Asthma and respiratory tract infections are also potential causes of dyspnea in this cohort [5]. Diagnostic guidelines for PPCM include echocardiography for ejection fraction, BNP levels, ECG for arrhythmias, and cardiac MRI for accurate ejection fraction and chamber size measurements [6]. PPCM management includes heart failure medications (loop diuretics, hydralazine/nitrates, beta-blockers, digoxin pre-delivery, and ACE inhibitors, ARBs, aldosterone antagonists, sacubitril, ivabradine postpartum) and anticoagulants like low molecular weight heparin [1]. Bromocriptine, a dopamine agonist, is linked to positive outcomes in PPCM due to its effect on prolactin [7]. Some studies suggest the use of CardioMEMS on PPCM patients who are still pregnant to better monitor the progress of their condition and better administer their treatment regimen [8]. PPCM has a more favorable rate of recovery than in other types of Heart failure with reduced ejection fraction with most recoveries occurring within 6 months [1]. Full recovery is considered as a restoration of the Ejection Fraction to ≥ 50%. Partial recovery is defined as an improvement of at least 10% [9]. An incidence of the condition creates a need for risk evaluation for future pregnancies [10]. The patient's asthma history delayed her PPCM diagnosis, potentially complicating her course. She showed partial recovery over two years, with an ejection fraction of 35-40%. This case underscores the need to consider cardiac pathologies in peripartum women with chronic respiratory conditions and refractory dyspnea. Early use of cardiac biomarkers and imaging can reduce misdiagnosis and improve outcomes. Healthcare providers should maintain a high suspicion of PPCM in peripartum women with dyspnea.
Conclusion
This case emphasizes the need for heightened clinical vigilance in peripartum women with dyspnea, particularly those with asthma or other respiratory conditions. The delayed diagnosis of peripartum cardiomyopathy highlights the importance of considering cardiac causes in refractory dyspnea. Early use of echocardiography and biomarkers like BNP or NT-proBNP can enable timely intervention and improve outcomes. This case underscores the importance of multidisciplinary approaches and updated guidelines for prompt identification and treatment of PPCM.
References
- Davis M, Arany Z, McNamara D, et al. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. JACC, 2020; 75(2): 207–221.
- Sigauke FR, Ntsinjana H, Tsabedze N. Peripartum cardiomyopathy: a comprehensive and contemporary review. Heart Fail Rev, 2024; 29(6): 1261-1278.
- Bala R, Mehta S, Roy VC, Kaur G, de Marvao A. Peripartum cardiomyopathy: A Review. Revista Portuguesa de Cardiologia, 2023; 42(11): 917-924.
- Perea Rojas DM, Seni Hernandez CD, Rojas Torres IL, et al. Peripartum Cardiomyopathy: A Case Report of Mortality from a Rare and Potentially Fatal Condition. J Med Cases, 2024; 15(8): 171-179.
- Amit BH, Marmor A, Hussein A. Unilateral presentation of postpartum cardiomyopathy misdiagnosed as pneumonia. BMJ Case Rep, 2010; 2010: bcr0520103039.
- Rodriguez Ziccardi M, Siddique MS. Peripartum Cardiomyopathy. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- Takanaka H, Ono R, Kato H, Iwahana T, Miyahara T, Takahashi H, et al. Peripartum cardiomyopathy in patients with psychiatric disorders successfully treated with bromocriptine: Two case reports. J Cardiol Cases, 2023; 29(3): 136-139.
- Sharma R, Dunn MC, Tam Tam H, Shah SK. CardioMEMS as an aid to the management of a pregnant patient with peripartum cardiomyopathy: A case report. Eur J Obstet Gynecol Reprod Biol, 2024; 303: 279-281.
- Carlson S, Schultz J, Ramu B, Davis MB. Peripartum Cardiomyopathy: Risks Diagnosis and Management. J Multidiscip Healthc, 2023; 16: 1249-1258.
- Pachariyanon P, Bogabathina H, Jaisingh K, et al. Long-Term Outcomes of Women with Peripartum Cardiomyopathy Having Subsequent Pregnancies. JACC, 2023; 82(1): 16–26.

