Removal of Two Encrusted JJ Ureteral Stunts Using Ureteroscopy and Holmium-YAG Laser Lithotripsy
Safwate R*, Nachid A, Safieddine M, Kbirou A, Moataz A, Dakir M, Debbagh A and Aboutaeib R
Urology department, IBN ROCHD University hospital, Casablanca, Morocco
Received Date: 24/11/2024; Published Date: 30/12/2024
*Corresponding author: Safwate Reda, Urology department, IBN ROCHD University hospital, Casablanca, Morocco
Abstract
Ureteral stents are essential medical devices used to manage urinary obstructions caused by various conditions, including stones, tumors, and fibrosis. Despite their utility, prolonged stent retention due to patient non-compliance or inadequate follow-up can lead to serious complications, such as encrustation, infection, and kidney damage. This report presents a case of a 65-year-old patient with poorly managed diabetes who developed extensive calcification of two retained JJ stents, complicating their removal. Advanced endoscopic techniques, including multiple sessions of Holmium-YAG laser lithotripsy, were employed to successfully remove the encrusted stents and preserve renal function.
Keywords: Retained ureteral stent; Stent encrustation; Holmium-YAG laser lithotripsy; Endoscopic stent removal; Ureteroscopy
Introduction
A ureteral stent is a medical device commonly used to restore the functional patency of a non-draining ureter. It is typically indicated for cases of ureteral obstruction caused by stones, tumors, or fibrosis; postoperative inflammation following ureteral repair or anastomosis; and as a preventive measure in patients undergoing shock wave lithotripsy who are at high risk of obstruction due to large stones. Stent placement is usually performed by a urologist under fluoroscopic guidance to ensure proper positioning [1,2].
Before the procedure, patients are informed about the process, potential stent-related symptoms, and possible complications. Complications associated with ureteral stents include hematuria, urinary tract infections (UTIs), stent migration, and stent retention [3]. Among these, retained or forgotten stents are entirely preventable and often result from a combination of patient and surgical non-compliance. Retained stents can lead to serious, sometimes life-threatening complications due to encrustation or calcification [4].
Ureteral stents are occasionally forgotten for extended periods [5], with the risk of complications increasing as the indwelling time is prolonged [6]. Numerous case reports have detailed the complications associated with forgotten stents, which are typically managed through endoscopic removal procedures [7].
The management of encrusted retained stents often requires multimodal procedures, including extracorporeal shock wave lithotripsy (ESWL), ureteroscopy (URS), cystolithotripsy, and percutaneous nephrolithotomy (PCNL) [8].
We report the case of a 65-year-old patient with two encrusted JJ stents.
Case Report
We report the case of a 65-year-old patient, type 2 diabetic, poorly monitored, on oral antidiabetics, who had undergone a right nephrectomy 20 years ago for pyonephrosis on lithiasis pathology. He had a left JJ catheter inserted 4 years before coming to our department, in a hospital other than ours, for acute obstructive pyelonephritis, and had not been educated about his JJ catheter (duration, removal). He had consulted the emergency department for febrile left lower back pain, which revealed acute pyelonephritis on a calcified JJ whose removal was unsuccessful, necessitating the insertion of a second JJ. The patient was informed of the seriousness of his case, but was lost to follow-up due to his low socio-economic level, lack of education, non-proximity to the hospital and neglect of his condition.
The patient consulted us 18 months after the second JJ catheter had been inserted. A standard X-ray showed massive calcification of the 2 JJ catheters in the patient's body, with migration of one of the JJ catheters (Figure 1). An abdominopelvic CT scan was ordered, revealing a globular, enlarged left kidney (150x95x80 mm) with bumpy contours and 2 proximal ends of the 2 JJ probes surrounded by extensive, gross and homogeneous calcifications, more marked in the proximal urinary tract, following the pyelo-caliceal cavities, with moderately dilated pyelo-caliceal cavities; the distal end of the migrated JJ probe was located in the pelvic ureter, while the other in the bladder (Figure 2). A standard laboratory work-up revealed moderate anemia at 10.6 g/dL, moderate renal failure with glomerular filtration rate at 45 ml/min/1.73 m² (calculated using the MDRD formula) and Klebsiella Pneumoniae urinary tract infection.
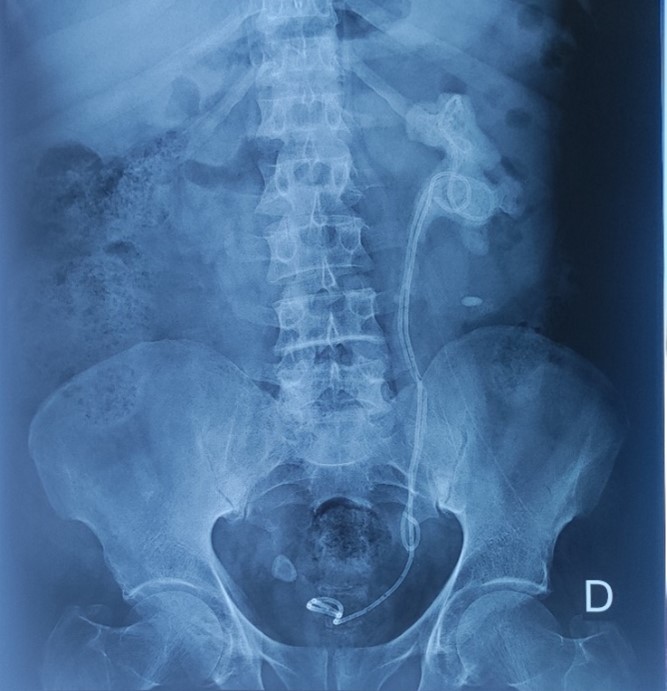
Figure 1: standard X-ray of the urinary tract showing the 2 calcified JJ catheters.
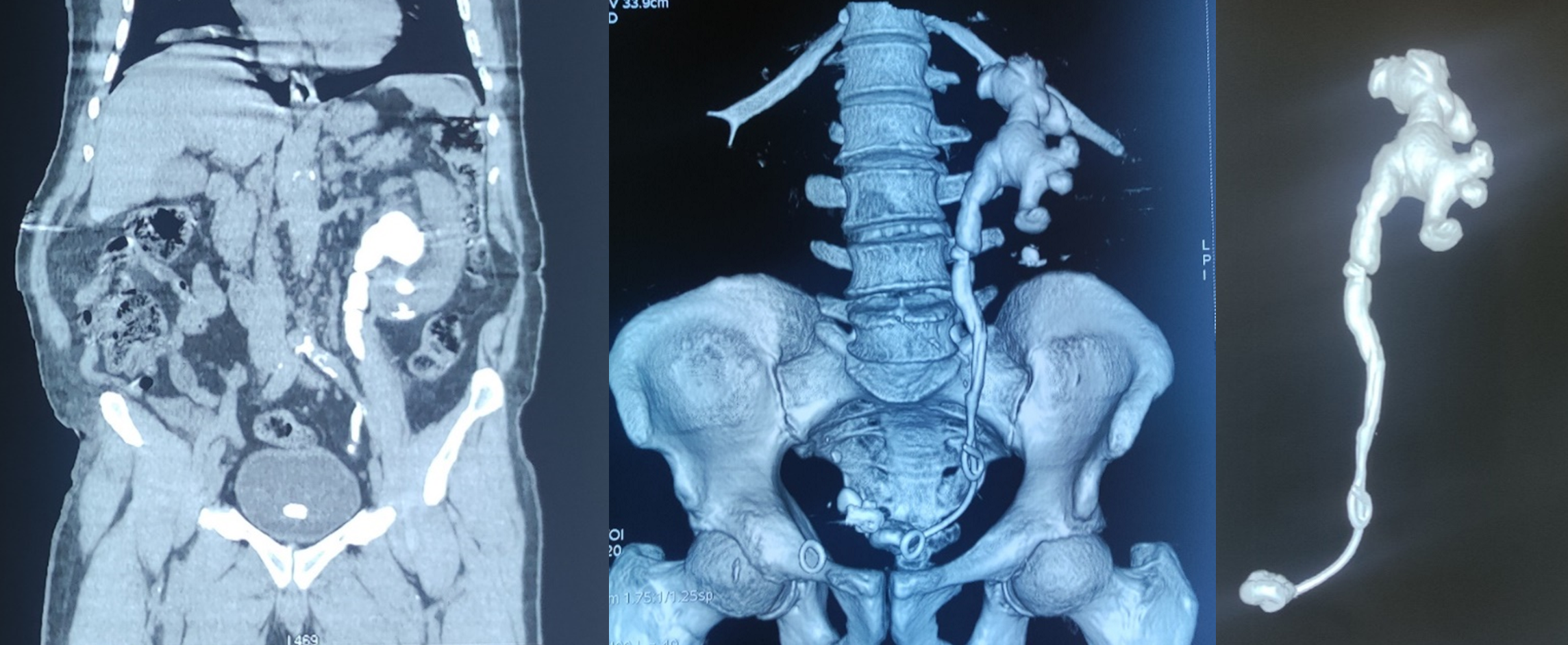
Figure 2: Abdominopelvic CT scan showing massive calcification of the 2 JJ probes with migration of one of them.
The therapeutic approach proposed to the patient was that of an endoscopic approach, by means of rigid and flexible URS, in order to free the JJ probes initially and then fragment the residual stones, recourse to PCNL or surgery would be if major difficulty of access or manipulation in order to preserve the single kidney as much as possible. The patient underwent a first Holmium-YAG (Ho-YAG) laser fragmentation with fragmentation of the JJ catheters down to the lumbar ureter, then a JJ catheter was left in place and the patient was not declared discharged from hospital for fear that he would be lost to follow-up again. The second fragmentation was performed 10 days later and was easier than the second, thanks to the JJ catheter left in place, which drained the kidney well and prepared the ureter, freeing up the rest of the ureter for access to the renal pelvis allowing ureteral access sheath passage. The 3rd fragmentation was performed using a flexible URS and enabled total liberation of the 2 JJ catheters; the residual stones were subsequently fragmented.
Discussion
Since their introduction in 1967, ureteral stents have remained one of the most commonly used tools by urologists for relieving renal and ureteral obstruction [7]. Regardless of the reason for stent placement, patient education, clear postoperative instructions, and meticulous record-keeping to ensure timely removal are essential to avoid prolonged stent retention.
Long-term retention of stents can result in complications such as pain, encrustation, obstruction, UTIs, and even loss of kidney function. Encrustation is a multifactorial process influenced by biofilm formation, patient-specific risk factors (e.g., a history of stones, UTIs, or pregnancy), and the properties of the stent material. It occurs due to the deposition of calcium oxalate or uric acid on the stent’s surface. Importantly, the duration of stent indwelling is a critical factor, with some studies showing that the rate of encrustation increases exponentially over time [7].
Periodic replacement or timely removal of ureteral stents is crucial to prevent complications such as encrustation, which increases with extended indwelling time. Research shows encrustation rates rise from 9.2% at less than six weeks to 76.3% beyond 12 weeks. Early removal, typically within three months, is recommended to avoid severe complications. Studies also highlight removal challenges, with 42.8% of stents becoming difficult to remove after four months and cases of irremovable stents after prolonged use, averaging 20 months [4,9–11].
The radio-opaque nature of ureteral stent encrustation makes standard kidney, ureter, and bladder (KUB) X-rays the first-line imaging modality for detection. However, ultrasound or CT scans are often utilized subsequently to precisely locate the encrustation relative to the ureter and/or stent. Once the extent of encrustation is identified, grading systems are used to assess the complexity of removal, followed by the application of a treatment algorithm to determine the most appropriate surgical technique [12].
The Forgotten, Encrusted, Calcified (FECal) System categorizes stent encrustation into five grades. Grade I represents minimal encrustation confined to the distal stent pigtail, while Grade V indicates extensive circumferential encrustation encasing the entire stent. The proposed treatment options range from cystoscopy and stent removal for minor encrustations to more invasive procedures such as ESWL, PCNL, cystolitholapaxy, URS, or combinations of these for severe cases. In instances where renal function is reduced to <20%, nephrectomy may be necessary [8].
The KUB System evaluates encrustation based on severity and location within the urinary tract [13]. A recently published treatment algorithm integrates both the FECal and KUB Systems, providing a comprehensive approach to managing stent encrustations [12].
In our case, and because of the patient's condition (single kidney), the endoscopic approach was chosen because it causes less damage and follows the natural urine pathway. Urologists with advanced training in endourology can typically manage forgotten double-J stents using endoscopic techniques, reserving open surgery as a last resort if endoscopic procedures are unsuccessful [4].
The removal of retained and encrusted ureteral stents is often surgically challenging, requiring various multimodal approaches to ensure patients are free of both stents and stones. Despite the prevalence of this issue, the available evidence for optimal treatment mainly consists of case reports and small series. While no universally accepted guidelines exist, several authors have proposed algorithmic approaches to facilitate successful stent removal.
Retained ureteral stents often necessitate multiple endourological procedures for removal, with reported averages ranging from 2 to 4.2 procedures to achieve a stent- and stone-free outcome [14]. However, single-step approaches have also demonstrated success. These methods typically involve retrograde URS combined with Ho-YAG laser lithotripsy to manage stent calcifications or fragment the stent itself, creating more space in the ureter for instruments. In a series of 36 patients, URS successfully removed encrusted stents in a single anesthetic [10].
The use of smaller URS has been shown to reduce the risks of mucosal injury, ureteral perforation, and ureteral avulsion, while potentially eliminating the need for PCNL, which is associated with higher morbidity and complication rates [4].
The development of flexible URS and Ho:YAG lasers has improved stent removal by enabling better observation and manipulation in the renal pelvis. While rigid URS can effectively remove proximal encrustation, it carries the risk of ureteral injury, making flexible URS with ureteral access sheath (UAS) a safer choice after confirming no adherence to the middle ureter.
Preoperative stenting is beneficial for ureter dilation, allowing for the insertion of larger caliber UAS and facilitating URS. UAS also reduces intrarenal pressure, improves irrigation flow, shortens operative time, and aids in retrieving stone fragments. Inserting a ureteral stent beside an encrusted one before URS is particularly helpful for drainage, dilation, and safe removal, especially in cases with significant encrustation. This approach minimizes risks such as ureteral stricture and optimizes surgical outcomes [15].
Despite the success of URS in many cases, a percutaneous approach may still be required for patients with significant proximal coil stone burdens and large renal stones. The degree of proximal loop encrustation has been correlated with a higher likelihood of requiring PCNL, multiple procedures, and an increased risk of surgical complications [16].
In a multicenter retrospective study by Pais et al., involving 38 renal units undergoing PCNL for encrusted ureteral stent removal with a mean dwell time of 28.2 months, PCNL alone was sufficient in only 21% of cases. Adjunctive procedures were often necessary, either during the PCNL or as separate operations, to ensure complete stent removal [16].
The "Tri-Glide" technique is an innovative approach for managing complex encrusted stent extractions. Its key advantage lies in modified patient positioning, enabling easier guidewire passage in cases of severe shaft encrustation (retrograde or antegrade fashion). This method is especially effective when stent coils cannot fully uncurl or when severe encrustation or ureteral narrowing prevents ureteroscope advancement [17].
To prevent the serious consequences of missed ureteral stents, several strategies have been developed:
- Advances in Stent Design and Materials: Modern stents are made from biocompatible materials like silicone and nitinol. Research is underway on biodegradable materials such as chitosan and polyglycolic acid to address complications from long-term use. Innovations in stent coatings and designs aim to reduce infection, encrustation, and pain, though widespread adoption of these materials will take time.
- Electronic Tracking Systems: Computerized databases with reminder systems, like the “traffic-light” method, improve stent management. Compared to manual logbooks, these systems significantly reduce cases of prolonged stent insertion caused by missed appointments or follow-up errors.
- Patient Education and Reminders: Educating patients about the risks of stents and providing direct reminders help mitigate follow-up lapses. Strategies include wristbands with barcodes for tracking, mobile applications for reminders, and online platforms to monitor appointments and reduce complications due to forgotten stents.
These methods collectively improve patient safety and stent management, minimizing risks associated with delayed removal [18–20].
Conclusion
Over the years, various interventions have been introduced to reduce the incidence of forgotten ureteral stents, but none have been entirely successful in eliminating this preventable complication. Developing new and more effective strategies to ensure timely stent removal is imperative. Ultimately, adhering to the principle of "do no harm" requires both patients and physicians to take responsibility. Patients must be adequately educated about their stents, and physicians must ensure clear communication and proper follow-up to prevent life-threatening complications from ureteral stent retention.
Financial support and sponsorship: None.
Conflicts of interest: There are no conflicts of interest.
References
- Fiuk J, Bao Y, Calleary JG, Schwartz BF, Denstedt JD. The Use of Internal Stents in Chronic Ureteral Obstruction. Journal of Urology, 2015; 193(4): 1092‑1100.
- Pengfei S, Min J, Jie Y, Xiong L, Yutao L, Wuran W, et al. Use of Ureteral Stent in Extracorporeal Shock Wave Lithotripsy for Upper Urinary Calculi: A Systematic Review and Meta-Analysis. Journal of Urology, 2011; 186(4): 1328‑1335.
- Paick SH, Park HK, Oh SJ, Kim HH. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology, 2003; 62(2): 214‑217.
- Kawahara T, Ito H, Terao H, Yoshida M, Matsuzaki J. Ureteral Stent Encrustation, Incrustation, and Coloring: Morbidity Related to Indwelling Times. Journal of Endourology, 2012; 26(2): 178‑182.
- Sali GM, Joshi HB. Ureteric stents: Overview of current clinical applications and economic implications. Int J of Urology, 2020; 27(1): 7‑15.
- Polat H, Yücel MÖ, Utangaç MM, Benlioğlu C, Gök A, Çift A, et al. Management of Forgotten Ureteral Stents: Relationship between Indwelling Time and Required Treatment Approaches. Balkan Med J, 2017.
- Adanur S, Ozkaya F. Challenges in treatment and diagnosis of forgotten/encrusted double-J ureteral stents: the largest single-center experience. Renal Failure, 2016; 38(6): 920‑926.
- Acosta-Miranda AM, Milner J, Turk TMT. The FECal Double-J: A Simplified Approach in the Management of Encrusted and Retained Ureteral Stents. Journal of Endourology, 2009; 23(3): 409‑415.
- Kawahara T, Ito H, Terao H, Yamashita Y, Tanaka K, Ogawa T, et al. Ureteral Stent Exchange under Fluoroscopic Guidance Using the Crochet Hook Technique in Women. Urol Int, 2012; 88(3): 322‑325.
- Xu C, Tang H, Gao X, Gao X, Yang B, Sun Y. Management of forgotten ureteral stents with holmium laser. Lasers Med Sci, 2009; 24(2): 140‑143.
- Bultitude MF, Tiptaft RC, Glass JM, Dasgupta P. Management of encrusted ureteral stents impacted in upper tract. Urology, 2003; 62(4): 622‑626.
- Tomer N, Garden E, Small A, Palese M. Ureteral Stent Encrustation: Epidemiology, Pathophysiology, Management and Current Technology. Journal of Urology, 2021; 205(1): 68‑77.
- Arenas JL, Shen JK, Keheila M, Abourbih SR, Lee A, Stokes PK, et al. Kidney, Ureter, and Bladder (KUB): A Novel Grading System for Encrusted Ureteral Stents. Urology, 2016; 97: 51‑55.
- Murthy KVR, Reddy SJ, Prasad DV. Endourological Management of Forgotten Encrusted Ureteral Stents. Int braz j urol, 2010; 36(4): 420‑429.
- Kawahara T, Ito H, Terao H, Ogawa T, Uemura H, Kubota Y, et al. Encrusted Ureteral Stent Retrieval Using Flexible Ureteroscopy with a Ho: YAG Laser. Case Reports in Medicine, 2012; 2012: 1‑4.
- Pais VM, Chew B, Shaw O, Hyams ES, Matlaga B, Venkatesh R, et al. Percutaneous Nephrolithotomy for Removal of Encrusted Ureteral Stents: A Multicenter Study. Journal of Endourology, 2014; 28(10): 1188‑1191.
- Perez A, Nolte AC, Maurici G, Small AC, Liem SS, Pereira JF, et al. The “Tri-Glide” Technique: A Case Report on a Novel Intraoperative Approach for Removal of Retained and Encrusted Ureteral Stents. Duchene D, éditeur. Case Reports in Urology, 2022; 2022: 1‑4.
- Mosayyebi A, Manes C, Carugo D, Somani BK. Advances in Ureteral Stent Design and Materials. Curr Urol Rep, 2018; 19(5): 35.
- Davis NF, Murray G, O’Connor T, Browne C, MacCraith E, Galvin D, et al. Development and evaluation of a centralised computerised registry for ureteric stents: completing the audit cycle. Ir J Med Sci, 2017; 186(4): 1057‑1060.
- Ziemba JB, Ludwig WW, Ruiz L, Carvalhal E, Matlaga BR. Preventing the Forgotten Ureteral Stent by Using a Mobile Point-of-Care Application. Journal of Endourology, 2017; 31(7): 719‑724.

