Multiple Hydatidosis with Pancreatic Localization
Bachar A, Benzidane K*, Essaidi Z, Elabbassi T and Lefriyekh R
Department of General Surgery, IBN ROCHD University Hospital Center, Casablanca, Morocco
Received Date: 08/11/2024; Published Date: 11/12/2024
*Corresponding author: Benzidane K, Department of General Surgery, IBN ROCHD University Hospital Center, Casablanca, Morocco
Abstract
Hydatidosis is an anthropozoonosis caused by the infestation of humans by the larval form of the taenia Echinococcus Granulosis. It presents a health problem in some countries, including Morocco, which is an endemic country [1,2]. The site of implantation and development to interest any organ, including the pancreas, which is a rare site [3]. We report a case of multiple hydatidosis with thoracic, hepatic, pancreatic, splenic and peritoneal localization.
Keywords: Hydatid cyst; Pancreas; Liver; Peritoneal hydatidosis
Introduction
Hydatidosis is an anthropozoonosis caused by the infestation of humans (who are an accidental host) by the larval form of the taenia Echinococcus Granulosis. It presents a health problem in some countries of the Mediterranean basin, particularly in North Africa, including Morocco, which is an endemic country with 1500 cases/year, this is explained by the persistence of traditional livestock farming [1,2].
The site of implantation and development to interest any organ, including the most common hepatic and pulmonary. However, pancreatic localization is a rare site [3].
The diagnosis is based on a combination of clinical, radiological and biological elements given the variety of pancreatic cyst involvement [4].
We report a case of multiple hydatidosis with thoracic, hepatic, pancreatic, splenic and peritoneal localization.
Case Report
We report the case of a 47-year-old patient, whose risk factors are residence in a rural environment with contact with dogs and farm and family animals (father and brothers treated for KHF). Operated on 2 times for peritoneal hydatidosis in 1997 and 2012. Admitted to the general surgery department at the CHU IBN ROCHD for recurrence revealed by abdominal pain of the heaviness type without any notion of jaundice, or other abdominal or thoracic symptoms, a picture evolving for 1 month in a context of conservation of general condition. The clinical examination on admission found a patient in good general condition, anicteric and apyretic. The abdominal examination revealed a scar of the median laparotomy above umbilical with the presence of a mass on the right flank of 10 cm in length, painless, renal and without inflammatory signs in front of it. The rest of the clinical examination, particularly thoracic examination, was without abnormality.
On the thoraco-abdomino-pelvic CT scan:
Dysmorphic liver site of several hydatid cysts sparing almost no segments, some of which are located at the straddle level of segments VIII and VII and VIII and V, encompassing the inferior vena cava which is laminated in front of it but normally remains opacified.
The spleen is the site of two cystic formations, measuring 59 x 35 mm and 86 x 62 mm.
Presence of several intraperitoneal cystic formations, the largest of which is located on the right flank with a thin wall and multi-vesicular content, measuring 104x95x95 mm.
Minimal dilation of the main bile duct and the intrahepatic bile ducts in relation a priori to a compressive effect of the exophytic cyst of the caudate lobe on the lower bile duct.
Cystic formation centered on the right cardiophrenic angle with subpulmonary extension compatible with a type III hydatid cyst.
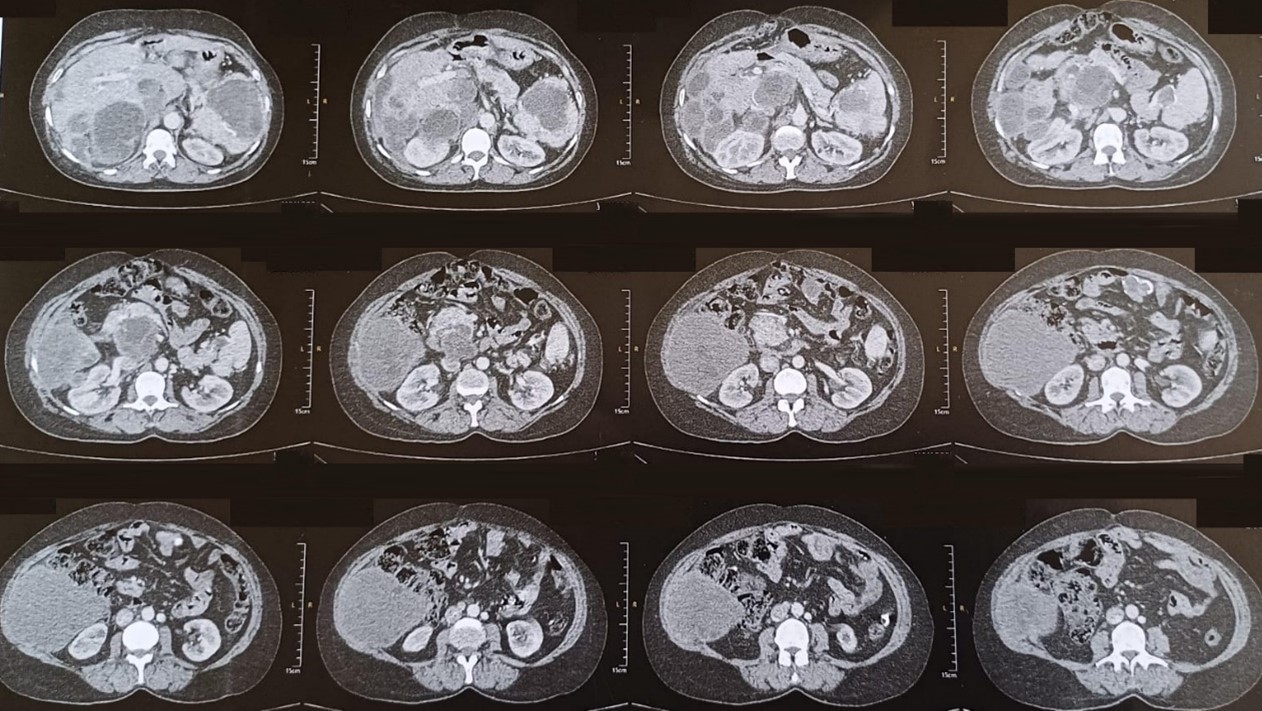
Figure 1: CT scan images.
Biologically, the complete blood count was abnormal, including normal white blood cell and eosinophilic levels. The cholestasis balance in particular the PAL and GGT were slightly increased with a normal bilirubin level. The hydatid serology was positive.
The management in consultation with the thoracic surgeon was to start with the abdominal floor first.
The patient started taking antihelminthic drugs before surgery with a dose of 800 mg twice a day, 5 days before surgery.
The patient was approached through a median incision above and below umbilical.
Intraoperative exploration objectified:
Hydatid cyst at the expense of the wall of the transverse colon of 4 cm, splenic of 10 cm, right parieto-colic groove of 15 cm, 2 hepatic hydatid cysts, one straddling segments II and III of 7 cm and one at the level of segment VII of 5 cm.
A hydatid cyst at the expense of the cephalic parenchyma of the head of the pancreas of 5 cm with absence of a cystic-ductal fistula.
All of these hydatid cysts were type III.
The surgical procedure was the resection of the protruding dome of all the hydatid cysts with drainage of the residual pancreatic, splenic, 2 hepatic cavities and the right parieto-colic gutter by large caliber Salem probes.
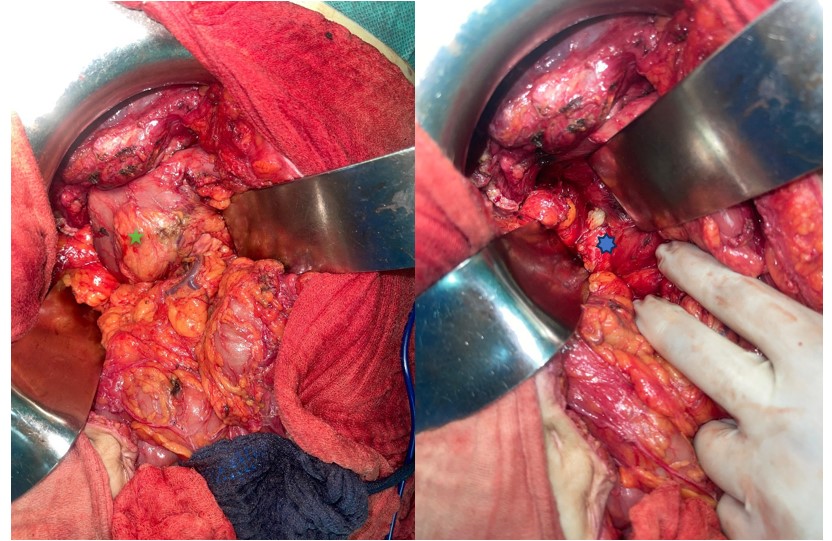
Figure 2: Intraoperative image of the pancreatic hydatid cyst.
(Green Star) ---> Duodeno-pancreatic block
(Blue Star) ---> Hydatid cyst at the expense of the head of the pancreas
The post-operative follow-up was simple. Feeding was authorized the day before the procedure with resumption of antihelminthic treatment the next day with the same dosage. The drains bringing back serous fluid, not exceeding 100 cc each, were removed on day 5 postoperatively. The patient was declared discharged on day 5 postoperatively on Albendazol with control of blood count and liver function on day 10 of treatment.
Discussion
Hydatid cyst, hydatid echinococcosis or hydatidosis, is a parasitic disease caused by the eggs of a tapeworm called Echinococcus granulosus. It belongs to the larval cestodes. It is a complex zoonosis affecting many species of animals. It accidentally affects humans, who are an intermediate host in the helminthiasis cycle [5].
Pancreatic localization is rare, representing 0.2 to 2% [6] and the cyst has an intraparenchymal development in 35% of cases and peripheral development in 65% of cases [7,8].%
Pancreatic localization is isolated in 91% of cases. The site is most often cephalic in 57% of cases, corporeal in 24% of cases and caudal in 19% of cases [9].
The route taken by the parasite is the superior mesenteric artery after passage of both hepatic and pulmonary or local filters from the liver through the pancreato-biliary ducts [7,10,11]
In the pancreas, the cyst gradually increases in size, ranging from a few millimetres to more than 20 cm, pushing back the pancreatic parenchyma, compressing and then eroding the surrounding organs. Thus, KHP has no specific clinical signs and the symptomatology depends on the location and size of the cyst [8,12,13].
Epigastralgia is the most common reason for consultation. Certain progressive complications may suggest the presence of a hydatid cyst of the pancreas, such as obstructive jaundice in the case of cysts located in the head of the pancreas, cyst abscess, chronic pancreatitis, rupture of the cyst in the intra-abdominal or retroperitoneal cavity, fistulization of the cyst in one of the neighboring organs and segmental portal hypertension. Mesenteric infarction has also been documented as a consequence of thrombosis of the superior mesenteric artery by compression exerted by the hydatid cyst. In addition, cyst fistulization in the Wirsung duct can lead to recurrent episodes of acute pancreatitis or even wirsungorrhagia [13].
Ultrasound, computed tomography and magnetic resonance imaging have no difficulty in recognizing the pancreatic cystic lesion, but the difficulty is to link this lesion to the hydatid disease [9].
Radiological features that distinguish hydatid cysts from other cystic lesions of the pancreas are the presence of curvilinear calcifications of the cyst wall, the presence of daughter vesicles, debris known as hydatid sand, septa, detachment of the hydatid membrane, or association with other more obvious hydatid cyst (liver) locations [11,14].
In the event that there is still a diagnostic doubt, the use of endoscopic ultrasound is of great benefit because it allows a better study of the cystic content [15].
A positive hydatid serology usually confirms hydatid disease, but the percentage positivity seems lower compared to the hepatic and pulmonary location. However, the negativity of serology does not eliminate the hydatid nature of a pancreatic cystic mass [16].
Diagnostic needle aspiration is formally contraindicated because of the risk of retro- or intraperitoneal dissemination [17]
The differential diagnosis is made with other macrocystic tumors of the pancreas. The pseudocyst is distinguished from the hydatid disease by the absence of a clean wall. Cystadenoma and cystadenocarcinoma are characterized by the enhancement of the intracystic edges and septa after injection of the contrast agent with a CT scan [18].
The anamnesis plays a crucial role in the diagnosis of hydatidosis by looking for risk factors such as contact with dogs and the socio-professional context. The examination also makes it possible to look for the notion of previous intervention on hepatic hydatidosis and the existence of an acute accident suggestive of the rupture or cracking of a hydatid cyst [19].
Thus, the comparison of preoperative data, in particular the anamnesis which plays a crucial role in the diagnosis of hydatidosis by looking for risk factors such as contact with dogs and the socio-professional context, the clinics, radiological (ultrasound, computed tomography, MRI and possibly endoscopic ultrasound) and serology allow, in the majority of cases, to confirm the hydatid nature of a pancreatic cystic mass [13].
The treatment of hydatid cyst of the pancreas is surgical. The location of the cyst and the presence or absence of a ductal cystic fistula make it possible to dictate the surgical action to be taken [8]. Thus, for hydatid cysts of cephalic location, resection of the protruding dome with or without epiplooplasty and drainage of the residual cavity by a large drain given the non-negligible risk of postoperative pancreatic fistula, is the therapeutic choice [9]. Cephalic duodenopancreatectomy seems to be a disproportionate procedure in the face of this benign parasitic condition [20].
Some authors suggest the systematic administration of Sandostatin® at induction and postoperatively for seven days for preventive purposes, since the residual cavity of a hydatid cyst of the pancreas is considered to be the equivalent of a slice of pancreatic section, with a non-negligible risk of postoperative pancreatic fistula. [13].
In the case of a cystic-ductal fistula, a cystic-digestive anastomosis such as a cystic-gastric or cystic-duodenal anastomosis, or a cyst-jejunal anastomosis on a Y-loop should be preferred to external drainage or resection of the protruding dome because of the resulting complications. In case of friable pancreatic tissue not showing up on anastomosis, a root canal suture on a stake drain is indicated [9,21].
For corporeo-caudal localization, a left pancreatectomy removes the cyst and sutures the pancreas into healthy tissue. Resection of the protruding dome is considered for large cysts adhering to neighbouring organs with the risk of dissection which can be dangerous [13].
The introduction of albendazole is indicated in cases of intraoperative rupture of the cyst or multiple hydatidosis [22].
Conclusion
In conclusion, the pancreatic localization of the hydatid cyst remains less frequent in the case of multiple abdominal localization and all the more rare as a primary localization. The diagnosis is essentially based on imaging, serology and the search for risk factors such as contact with dogs and the socio-professional context or previous intervention such as our patient.
Management is based on the presence or absence of a cystic pancreatic fistula and the standard treatment in the absence of it and the resection of the protruding dome with drainage of the residual cavity.
References
- Venara Aurélien, Mehinto Delphin K, Lermite Émilie, Chabasse Dominique, Hamy Antoine, Arnaud Jean-Pierre. Unusual primary locations of hydatid cyst. Med. Press, 2011; 40(4): 438-442.
- Epidemiological bulletin. The retrospective survey on hydatidosis, period 1980-1992. 1st quarter 1995, 1996.
- Elfazazi H, Kouach J, Babahabib A, Oukabli M, Hafidi MR, Salek G, et al. Pelvic primary hydatid cyst. Imagery of the Woman, 2010; 20(2): 438-442.
- El Jai SR, Boufettal R, Farah RH, Chehab F. Pancreatic hydatid cyst: about a case. Pan Afr Med J, 2015; 21(1): 273.
- Rey P, Mbaye P-S, Debonne J-M, Klotz F. Parasitic liver. EMC – Hepatology, 2004; 1(2): 69-81.
- Pouget Y, Mucci S, O'Toole D, Lermite E, Aubé C, Hamy A. Recurrent acute pancreatitis revealing a hydatid cyst of the pancreas. Rev Med Internal, 2009; 30(4): 358-360.
- Bouasker I, Zoghlami A, Ben Achour J, Najah H, Bedoui R, Hani ML, et al. Hydatid cysts of the pancreas, report of two cases. Tunis Médicale, 2009; 87(2):155-158.
- Khiari A, Mzali R, Ouali M, Kharrat M, Kechaou MS, Beyrouti MI. Hydatid cyst of the pancreas: a propos of 7 cases. Ann Gastroent érologie Hépatologie, 1994; 30(3): 87-91.
- Fadil A, Ait Bolbarod A, El Fares F. Hydatid cyst of the pancreas. About an observation. Ann Chir, 2000; 125(2): 173-175.
- Oruc MT, Kulacoglu IH, Hatipoglu S, et al. Primary hydatid cyst of the pancreas related to main pancreatic duct. A case report. Hepato Gastroenterology, 2002; 49: 383–384.
- Elmadi A, Khattala K, Elbouazzaoui A, Rami M, Labib I, Harandou M, et al. Journal of Pediatrics and Childcare, 2010; 23(4): 201-203.
- Wani RA, Malik A, Chowdri N, et al. Primary extrahepatic abdominal hydatidosis. Int J Surg, 2005; 3 (2): 125-127.
- Bedioui H, Chebbi F, Ayadi S, Daghfous A, Bakhtri M, Jouini M, et al. Primary hydatid cyst of the pancreas: diagnosis and surgical modalities: about three cases. Gastroenterol Clin Biol, 2008; 32(1):102-106.
- Zakari S, Ajana A, Dhobb OH, et al. Ultrasound aspects of the hydatid cyst of the pancreas: about 2 cases. Ann Radiol (Paris), 1984; 27(7): 607-613.
- Trabelsi S, Moussa A, Cherif I, et al. Usefulness of endoscopic ultrasonography for diagnosis between multivesicular hydatic cyst and serous cystadenoma of the pancreas: Case report. Tunis Med, 2005; 83(12): 785-788.
- Chammakhi-Jemli C, Mekaouer S, Miaoui A, Daghfous A, Mzabi H, Cherif A, et al. Acute pancreatitis revealing a hydatid cyst of the pancreas. J Radiol, 2010; 91(7-8): 797-799.
- Hedfi Mohamed et al. Hydatid cyst of the pancreas revealed by acute pancreatitis: about a case. Pan African Medical Journal, 2015.
- Muscari F, Suc B, Escat J, et al. Cystic tumors of the pancreas. J Chir, 2002; 139: 312–323.
- Laghzaoui Boukaid M, Bouhya S, Soummani A, Hermas S, Bennan O, Sefrioui O, et al. Pelvic hydatid cysts: about eight cases. Gynecol Obstet Fertil, 2001; 29(5): 354-357.
- Arnaud A, Sarles JC, Belkhodja C, Larabi B. Hydatid cyst of the pancreas: a propos of 2 cases. Chir M ém Académie Chir, 1991; 117(8): 607-612.
- Abid M, Guirat A, Ben Salah K, Khlif M. Hydatid cyst of the pancreas: an exceptional location. Arch Pediatr. 2010 Jul; 17(7):1056-1058.
- Elmadi A, Khattala K, Elbouazzaoui A, Rami M, Labib I, Harandou M, et al. Journal of Pediatrics and Childcare, 2010; 23(4): 201-203.

