FDG-Positive Lung Mass and Bilateral Hilomediastinal Lymphadenopaty - Francisella Tularensis Infection Mimicking Pulmonary Neoplasia
Aleksandrs Canajevs1,*, Frank Rassouli1, Regulo Rodriguez2 and Martin Brutsche1
1Department of Pneumology & Sleep Medicine Kantonsspital St. Gallen, St. Gallen, Switzerland
2Department of Pathology, Kantonsspital St. Gallen, St. Gallen, Switzerland
Received Date: 05/11/2024; Published Date: 09/12/2024
*Corresponding author: Aleksandrs Canajevs, Department of Pneumology & Sleep Medicine Kantonsspital St. Gallen, St. Gallen, Switzerland
Abstract
We present a case of a 69-year-old male non-smoker, referred for a PET-positive lesion in the left lower lobe and bilateral PET-positive hilomediastinal, infra- and supraclavicular lymphadenopathy. As lung cancer seemed to be the most likely diagnosis, a bronchoscopy with endobronchial ultrasound-guided transbronchial needle aspiration was performed. As tissue samples showed no malignant cells, a second bronchoscopy was performed, which again did not reveal malignancy. With granulocytic necrotizing inflammatory changes being the only histologic finding, an infectious cause had to be considered. Serologic testing for Francisella tularensis was performed and led to an initially unexpected diagnosis of pulmonary tularaemia.
Keywords: Tularaemia; Lung cancer; Pulmonary lesion
Introduction
Positron Emission Tomography (PET) provides significant advantages in the diagnosis and management of various medical conditions including malignant tumours and a broad spectrum of inflammatory diseases. We present a patient with a FDG-positive 33mm-diameter pulmonary mass and bilateral intrathoracic as well as infra- and supraclavicular lymphadenopathy, strongly suggestive of advanced lung cancer. An endoscopic ultrasound-assisted tissue sampling was performed twice. Contrary to our expectations, histology revealed no signs of malignancy but clear signs of acute granulocytic inflammation. Consecutive serologic testing revealed an acute pulmonary Francisella tularensis-infection.
Case Report
A 69-year-old man was referred to the pulmonology department of the Cantonal Hospital St. Gallen for further work up after a PET-CT had been performed and had revealed a 33 mm PET-positive mass in the posterobasal segment of the left lower lobe and bilateral PET-positive hilomediastinal, infra- and supraclavicular lymphadenopathy.
He originally had presented in the department of rheumatology about ten days prior with intermittent fever, unintended weight loss and night sweats. He had a history of rheumatoid arthritis and polymyalgia rheumatica. The patient, a lifelong farmer, had no history of smoking or lung disease. His father was diagnosed with “farmers lung” more than 20 years ago. The family history was otherwise not significant.
Vital signs and nutritional status at the time of presentation excluding the fever were normal. His medication included Tamsulosin for benign prostate hyperplasia and a vitamin-D-supplement. Up to 2 years ago, he was on methotrexate, which was discontinued due to thrombo- and leukopenia. Since, he did not have any immunomodulatory treatment.
Laboratory findings revealed a moderately elevated C-reactive protein (53 mg/l) and a mild thrombocytosis (378 G/l). White blood cell count, erythrocyte sedimentation rate, procalcitonin, liver enzymes, antinuclear antibodies, Ro- and La- antibodies were in normal ranges.
As the patient was residing in the rheumatology ward, a PET-CT was ordered to exclude malignancy or vasculitis. Upon receipt of the findings, a prompt referral to pulmonology was initiated. With lung cancer being the most likely diagnosis, the patient underwent a bronchoscopy with sonography-guided needle aspiration of the affected lymph nodes as well as a transbronchial biopsy of the lesion in the left lower lobe.
The initial histologic specimens obtained by EBUS-TBNA of the N4R, N7, N10L lymph node stations and a transbronchial biopsy of the posterobasal left lower lobe showed granulocytic-necrotic inflammation with no sign of malignant cells. After discussing the case in an interdisciplinary thoracic tumour board, a repeat bronchoscopy was conducted. The second tissue sampling with biopsy of the left main bronchus and again EBUS-TBNA of lymph node stations N2R and N7 revealed similar results with granulocytic, partially necrotic inflammation with elements of an epitheloid-histiocytic infiltration and again without signs of malignancy.
A thorough anamnesis was conducted and the patient revealed being bit by a mouse some days prior to the manifestation of symptoms. Serologic tests were positive for Francisella tularensis (IgG 120.9 IU/ml and IgM 201.2 IU/ml) and the respective diagnosis retained. We treated the patient with a four-week regimen of ciprofloxacin, upon which he fully recovered. A follow-up CT of the chest was performed approximately 2 months after the diagnosis. Both the pulmonary lesion and the lymphadenopathy were significantly less pronounced.
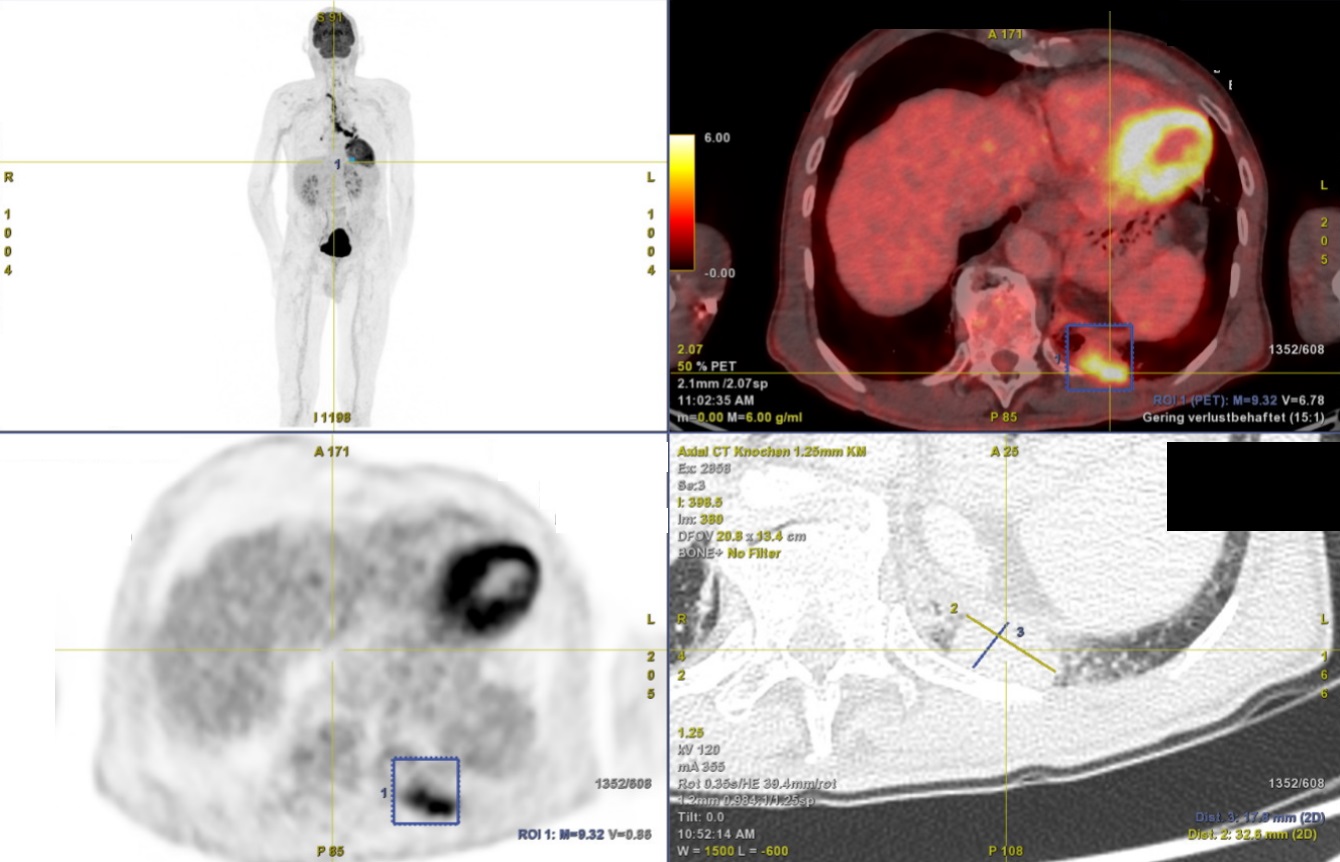
Figure 1: PET-CT and a CT scan of the chest showing a PET-positive mass in the posterobasal segment of the left lung (17.8mm x 32.6mm) and PET-positive lymph nodes.
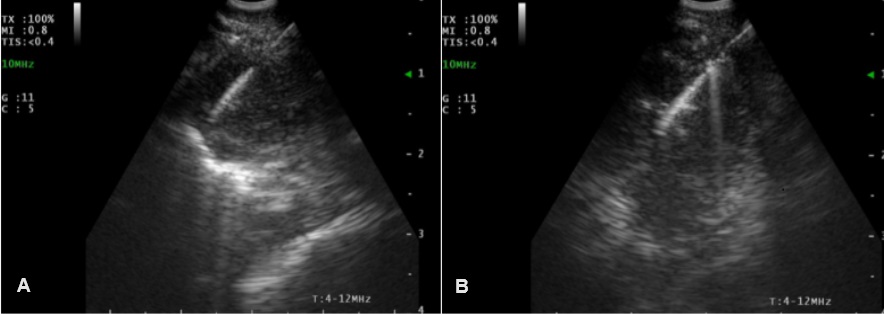
Figure 2: First bronchoscopy and endobronchial ultrasound-guided needle aspiration of enlarged lymph nodes; A: Station N4R, B: Station N7.
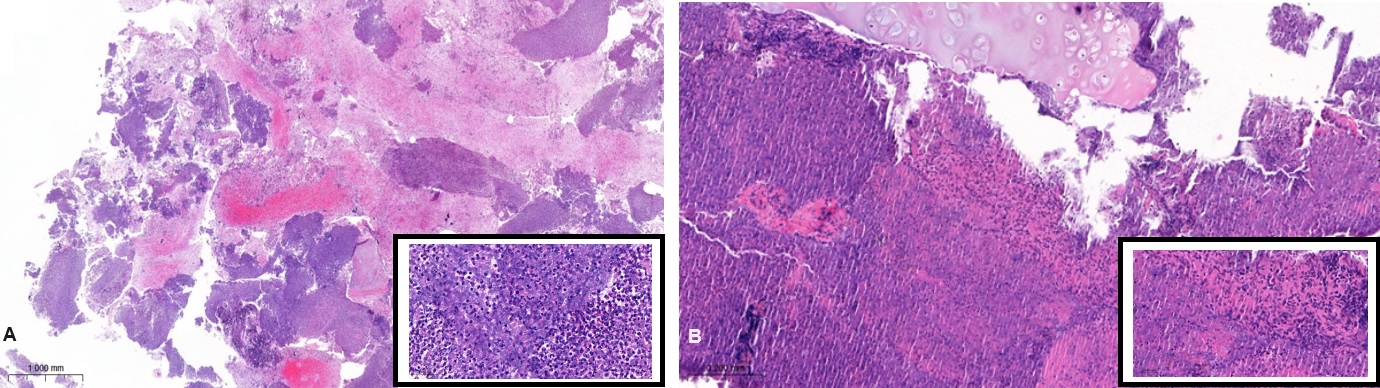
Figure 3: Hematoxilin-Eosin stains of EBUS-TBNA station N7 of the first (Panel A) and second (Panel B) bronchoscopy.
A (HE, 2.2x): granulocytic necrotic material/cartilage; B (HE 34.1x): necrosis with epithelioid histiocytic reaction.

Figure 4: CT scan showing significantly regressive pulmonary lesion 6 weeks after initiation of antibiotic treatment.
Discussion
Tularaemia is a zoonotic infection with clinical manifestations ranging from an asymptomatic infection to severe illness, in rare cases incluging septic shock. The manifestation of the disease is driven by the site of inoculation, the patients’ immune status and the strain of the bacterium [1].
F. tularensis-infection occurs throughout Europe, many parts of Asia and North America but are practically absent in the southern hemisphere [2]. Tularaemia is considered a rare disease, but seems to be on the rise in recent years in central Europe [3]. The rising prevalence may also in part be due to more countries making reporting new tularaemia cases compulsory.
Transmission usually occurs through contact to rodents, lagomorphs and tick bites. Contact to domestic cats may also play a role, especially in rural areas. It is possible to contract the disease by ingesting contaminated food or water.
One outbreak at an US-based correctional facility in 2011 was reported to be due to ingestion of contaminated ice [4]. Inhaling infected dust, hay and water vapour can also lead to infection.
Pulmonary tularaemia can be categorized as primary, when the bacterium is directly inhaled, or secondary in case of hematogenous spread to the lungs, as was the case in our patient. According to available studies [5], primary pulmonary manifestations of tularaemia were seen in up to 48% of all cases. The pulmonary form seems to more commonly affect patients with a compromised immune system.
The most virulent subspecies is F. tularensis subspecies tularensis. Some immunocompromised patients may progress to severe disease while being infected by the less virulent strains, for example the holarctica subspecies [6]. The emerging use of biological drugs such as TNF-alpha blockers is of concern as their use is considered as a risk factor for infection and disease severity.
Pulmonary tularaemia leads to an acute fibrinous and necrotizing pneumonia with a granulomatous reaction occurring 3 to 7 months after onset of symptoms [7]. A granulomatous reaction with or without giant cells or necrosis, similar to the inflammation pattern in cat-scratch disease has been described in lymph node samples [8]. F. tularensis cannot be satisfactory identified in tissue sections [9]. A histological report of “granulocytic-necrotizing” or “granulocytic-necrotizing-granulomatous” material in EBUS-TBNA or, in case of cryobiopsies or lymph node extirpation, a granulocytic or granulocytic-granulomatous inflammation is strongly suspicious of infection and should trigger further investigation.
If left untreated, pulmonary tularaemia can lead to respiratory failure and death. With this case, we would like to emphasise the importance of a complete patient history and prompt physicians to consider this rare disease as a differential diagnosis, especially in patients with a high risk of exposure to the pathogen and those with a compromised immune system. Furthermore, pulmonary tularaemia should be suspected in patients who have a rapid onset of unspecific and sometimes respiratory symptoms and CT-findings indicative of lung cancer in the absence of risk factors.
Disclosures
Author contributions: Aleksandrs Canajevs, main author, Frank Rassouli, supervisor of the endoscopic procedure and revision of the final report, Regulo Rodriguez histologic analysis of the specimens, Martin Brutsche scientific research supervisor.
Financial disclosure: none to report.
Previous presentation: none.
Informed consent was obtained for this case report.
References
- Bennet JE, Dolin R, Blaser MJ Francisella tularensis (Tularemia). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 9th ed, Elsevier, P.hiladelphia, 2020; p. 2759.
- Anders Sjöstedt. WHO Guidelines on Tularaemia, Geographical distribution of tularemia cases, World Health Organization, 2007; p. 5-6.
- Frischknecht M, Meier A, Mani B, Joerg L, Kim OC, Boggian K, et al. Tularemia: an experience of 13 cases including a rare myocarditis in a referral center in Eastern Switzerland (Central Europe) and a review of the literature, 2019; 47(5): 683-695. doi: 10.1007/s15010-019-01269-7.
- Brett ME, Respicio-Kingry LB, Yendell S, Ratard R, Hand J, Balsamo G, et al. Outbreak of Francisella novicida bacteremia among inmates at a louisiana correctional facility. Clin Infect Dis, 2014; 59(6): 826-833. doi: 10.1093/cid/ciu430.
- Tularemia Missouri. 2000-2007 Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep, 2009; 58(27): 744-748.
- Ting-Yi Su, Shian-Sen Shie, Ju-Hsin Chia, Ching-Tai Huang. Case Report of Low Virulence Francisella tularensis Presented as Severe Bacteremic Pneumonia. DOI: 10.1097/MD.0000000000003390.
- William D Travis, Thomas V Colby, Michael N Koss, Melissa L Rossado-de-Christenson, Nestor Luiz Müller, Talmadge E King Jr. Atlas of Nontumor Pathology, Non-Neoplastic Disorders of the Lower Respiratory Tract, First Series, Fascicle. American Registry of Pathology and the Armed Forces Institute of Pathology, Washington DC, 2002; p. 566.
- Atlas of Nontumor Pathology, First Series, Fascicle 7, Benign and Reactive Conditions of Lymph Node and Spleen, Dennis P. O’Malley, Tracy I. George, Attilio Orazi, Susan L. Abbondanzo, American Registry of Pathology and the Armed Forces Institute of Pathology, Washington DC, 2009; p.251-252.
- Volume I, Daniel H Connor, Francis W Chandler, David A Schwartz, Herbert J Manz, Ernest E Lack, et al. Utz. Pathology of Infectious Diseases. Appleton and Lange, Stanford, Connecticut, 1997; p. 871.

