Extraordinary Disease Control on Third-Line Treatment with Regorafenib in Metastatic Colon Cancer
M Omara1,2,*, Muhammad Farooq Latif1, Dina Hamza1, Swetha Kannan3, Wania M Akram3, Urooj Shahid3 and SH Tirmazy1
Department of Oncology, Dubai Hospital, Dubai, UAE
Received Date: 11/11/2024; Published Date: 02/12/2024
*Corresponding author: Dr. Mohamed Omara Ibrahim Hussien, Department of Oncology, Dubai Hospital, Al Khaleej st, Al Baraha, Dubai, UAE - Clinical Assistant Professor of Oncology, Mohammed Bin Rashid University of medicine and health sciences (MBRU), UAE
Abstract
Regorafenib, a multi-kinase inhibitor, was evaluated in the CORRECT trial for patients with metastatic colorectal cancer (mCRC) who progressed after standard therapies, demonstrating a median overall survival (OS) of 6.4 months compared to 5.0 months with placebo.
Keywords: Metastatic colon cancer; Regorafenib; Disease control; Third-line treatment; SBRT; Multi-kinase inhibitor; Case report
Case Presentation
We report a remarkable case of a 66-year-old gentleman with metastatic colon cancer and lung metastases who achieved extraordinary disease control with regorafenib as third-line treatment since 2018.
Clinical History
The patient's history began in August 2014 with chronic constipation. Colonoscopy revealed an ascending colon mass, and biopsy confirmed well-differentiated adenocarcinoma. A right hemicolectomy was performed, and histopathology showed pT3 pN2, K-RAS mutant, Her2 negative, and microsatellite stable (MSS) colonic adenocarcinoma. Postoperative CT scan revealed multiple lung lesions.
Treatment Timeline
- First-Line Treatment (2014-2016):
- FOLFOX/Avastin for 6 months followed by maintenance Xeloda/Avastin.
- In December 2016, He had lung disease progression and FOLFOX/Avastin rechallenge was reintroduced to the patient.
- Marginal lung disease progression in July 2017 and subsequent hematological toxicities led to a treatment break.
- Second-Line Treatment (March 2018-September 2018):
- FOLFIRI/Avastin initiated due to progression of lung lesions.
- In September 2018, PET scan showed further disease progression of all lung lesions.
- SBRT and Third-Line Treatment (November 2018-Present):
- SBRT was administered to lung lesions, and regorafenib was started at 160 mg/day.
- Dose reductions to 120 mg and then 80 mg/day were necessary due to abdominal pain and thrombocytopenia.
- The patient was continued on Regorafenib 80 mg with interval PET scans intially every 3-4 months then later every 6 months and were all showing excellent disease control untill 01/2024.
- January 2024: PET scan reported:
- Bilateral newly seen lung lesions with moderate metabolic activity at the right side with residual low grade metabolic activity at the previously reported bilateral basal lung patches, with mild right para tracheal hypermetabolic LNs further assessment is advised to verify their nature inflammatory vs active deposits.
We opted for continuation of same treatement and close follow up.
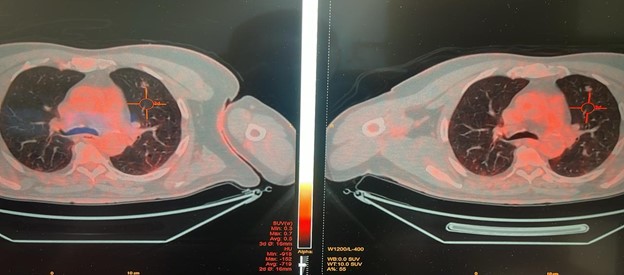
- April 2024: PET scan revealed:
- Both lungs showed irregular neoplastic lesions with increased metabolic activity at the right side suggestive for lung metastasis.
- Residual metabolic activity at the previously reported bilateral basal lung patches, with almost the same right para tracheal hypermetabolic LN, further assessment is advised to verify their nature inflammatory vs active deposits.
No newly seen suspicious hypermetabolic lesions could be detected elsewhere.
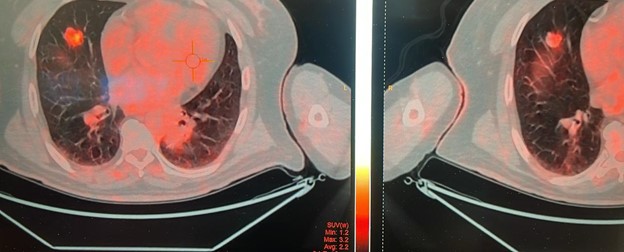
- A CT-guided biopsy confirmed metastatic adenocarcinoma of colonic origin. “ Attacged”.
- New NGS panel was performed and confirmed same findings: K-RAS mutant, Her2 negative, and microsatellite stable (MSS) – “ Attached”.
- We referred the patient for SBRT to lung lesions and continued on the same systemic treatment with Regorafenib.
- Recent PET Scan 09/2024 reported: Excellent partial response in the lung and no new lesions anywhere else. “ Attached”.
- The patient remains on regorafenib with reduced dose with extraordinary disease control and no significant toxicity.
Conclusion
This case underscores the potential efficacy of Regorafenib as a third-line treatment in metastatic colon cancer. The patient has achieved long-term disease control since 2018, highlighting the importance of personalized treatment plans and regular monitoring. Further studies are warranted to explore Regorafenib's role in similar clinical scenarios.

