Cholelithiasis in Paediatric Patient with Hereditary Spherocytosis. Is Intraoperative Cholangiogram Useful?
Irene Dieguez Hernandez-Vaquero*, Noela Carrera Guermeur, Salome Vincent Sorba, Lara Merino-Mateo, Hilda Josefa Ramirez Velandia and María Esmeralda Kuan Arguello
Paediatric Surgery Department, Hospital Universitario de Toledo, Avenida del Rio Guadiana, Spain
Received Date: 12/11/2024; Published Date: 29/11/2024
*Corresponding author: Irene Dieguez Hernandez-Vaquero, Paediatric Surgery Department, Hospital Universitario de Toledo, Avenida del Rio Guadiana, 45007, Toledo, Spain
Abstract
Most patients with hereditary spherocytosis will require surgical treatment (splenectomy and/or cholecystectomy) throughout their lives, making it a therapeutic challenge for the paediatric surgeon when required at an early age.
We present a 7-year-old male with hereditary spherocytosis and symptomatic cholelithiasis with repeated biliary colic (pain, nausea, dizziness). Furthermore, he associated an episode of mild acute cholecystitis with cholestasis (mild fever and pain without pale stools), therefore we decided to perform a delayed laparoscopic cholecystectomy. Due to the persistent elevation of bilirubin, mainly conjugated, we decided to associate Intraoperative Cholangiogram (IOC) to rule out Common Bile Duct (CBD) involvement.
Children with hereditary spherocytosis present manifestations such as cholelithiasis and splenomegaly with hypersplenism, which represent a challenge in clinical practice. In this case, simultaneous splenectomy was not indicated due to the absence of severe haematological symptoms. Small patients may benefit from IOC to rule out choledocholithiasis.
Keywords: Cholangiogram; Cholecystectomy; Hereditary spherocytosis; Splenectomy
Introduction
Hereditary Spherocytosis (HS) is the most common cause of hereditary haemolytic anaemia, with a prevalence in northern Europe and America of 1:1000-1:2000 patients [1]. In 75% of the cases there is a family history [2].
In the long term, the most common complications are cholelithiasis and splenomegaly with hypersplenism. The degree of anaemia reflects the severity of the haemolysis and determines the increase in the spleen’s size. Haemolysis can worsen during infections due to haemolytic crisis or aplastic crisis (Parvovirus B19 infection), even requiring blood transfusions or the administration of erythropoietin [1].
Although the evidence is limited, there is expert consensus on surgical management (splenectomy and/or cholecystectomy) in paediatric patients with HS [2].
Case Report
7-year-old male with HS diagnosed after first haemolytic crises during early childhood. Father and younger brother diagnosed with HS too. He presented with moderate anaemia, and had suffered 5 haemolytic crises, only one of them requiring a blood transfusion 3 years ago, the rest of them treated conservatively. He was adequately immunized for his age.
The patient consulted due to several episodes of intermittent abdominal pain in the right upper quadrant during the last 2 months, associated with dizziness and nausea but without concomitant jaundice, pale stool or fever. Physical exploration was normal except for 3 cm palpable splenomegaly. The initial assessment showed a total bilirubin of 2.76 mg/dL (conjugated 0.7 mg/dL), without elevated transaminases or GGT. Ultrasound revealed the recent appearance of multiple cholelithiasis without dilation (<6 mm) of the common bile duct (CBD), and splenomegaly, already known (Figure 1).
Paediatric haematologist consulted Surgery Department in order to perform laparoscopic splenectomy and cholecystectomy. After clinical assessment and in absence of severe haematological symptoms, we decided to perform laparoscopic cholecystectomy exclusively.
Prior to the surgical intervention, the patient presented with mild acute cholecystitis with low-grade fever, pain and jaundice and no pale stools. Ultrasound scan revealed minor thickening of the gallbladder wall without CBD dilation and minimal perivesicular free fluid. Blood analysis showed an increase in total bilirubin up to 14.52 mg/dL (conjugated 10.72 mg/dL) and an increase in AST (309 U/L), ALT (478 U/L) and GGT (205 U/L). During the acute episode, conservative management was adopted with antibiotic therapy (piperacillin-tazobactam IV 100 mg/kg/8h) for 7 days and, after resolution of the acute condition, a delayed laparoscopic cholecystectomy was scheduled after 4 weeks. Given that the patient had persistently conjugated hyperbilirubinemia (4.05 mg/dL; conjugated 2.23 mg/dL at time of surgery), despite no choledocholithiasis or CBD’s dilation detected in ultrasound, we decided to perform Intraoperative Cholangiogram (IOC), since Magnetic Resonance Cholangio-Pancreatography (MRCP) was not available.
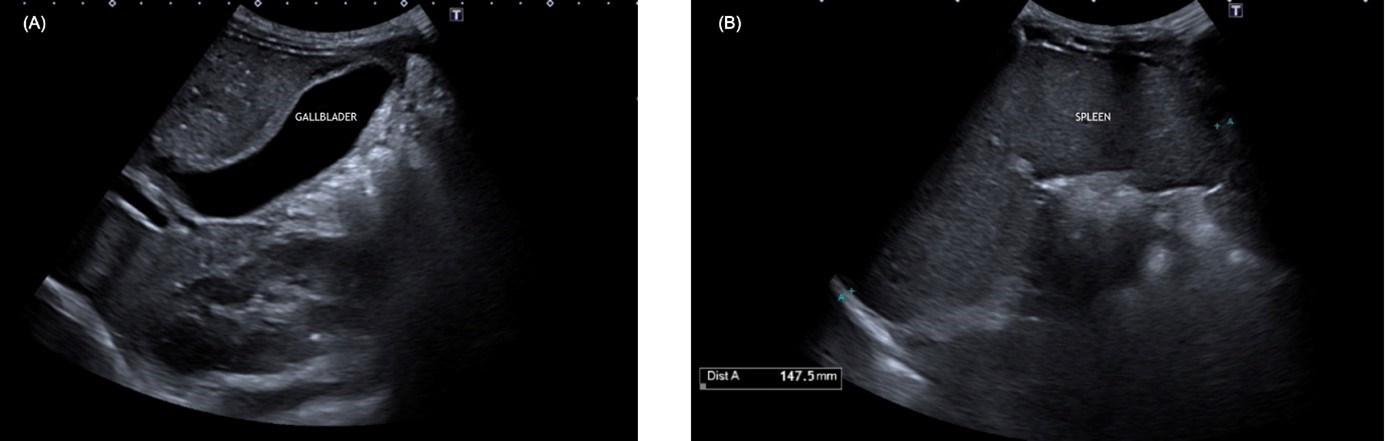
Figure 1: (A) Gallbladder with echogenic content with posterior acoustic shadow. (B) Homogeneous splenomegaly with a longitudinal diameter of 147.5mm.
Table 1: Degree of anaemia based on analytical parameters.
Management and Outcome
Preparation: prophylactic antibiotic therapy with IV amoxicillin-clavulanic (single dose 33 mg/kg) and bladder catheterization.
Position: patient in supine position with abducted legs.
Access: umbilical port for optic (5 mm and 30º) and three 5 mm accessory ports placed in the subxiphoid and supraumbilical midline, and in the midclavicular line on the right flank.
Procedure: after dissection and identification of the cystic artery and duct in the biliary pedicle, the artery was sealed with 10 mm Hem-o-lok ® and sectioned. Subsequently, the gallbladder was dissected until it was suspended exclusively from the cystic duct.
For IOC, the distal part of the cystic duct was ligated with an endoloop. Then a partial transection of the duct was performed with scissors in its anterior wall, and the orifice was dilated with a dissector. Finally, a 6 Fr. feeding tube was introduced through the right flank trocar to channel the cystic duct and instil water-soluble contrast (Omnipaque 300 mg Iodine/mL diluted to 50%) (Figure 2). Under fluoroscopic guidance, the presence of choledocholithiasis or other CBD anomalies were ruled out (Figure 3). After sealing the proximal end of the cystic duct with another Hem-o-lok ®, its section was completed and the gallbladder was exteriorized within a 5 mm endobag through the umbilical incision.
Postoperatively, 2 more doses of antibiotic prophylaxis with IV amoxicillin-clavulanic were administered. The patient remained afebrile and had good pain control with IV analgesia, followed by oral analgesia. He was finally discharged 48 hours later, after adequately restarting oral feeding.
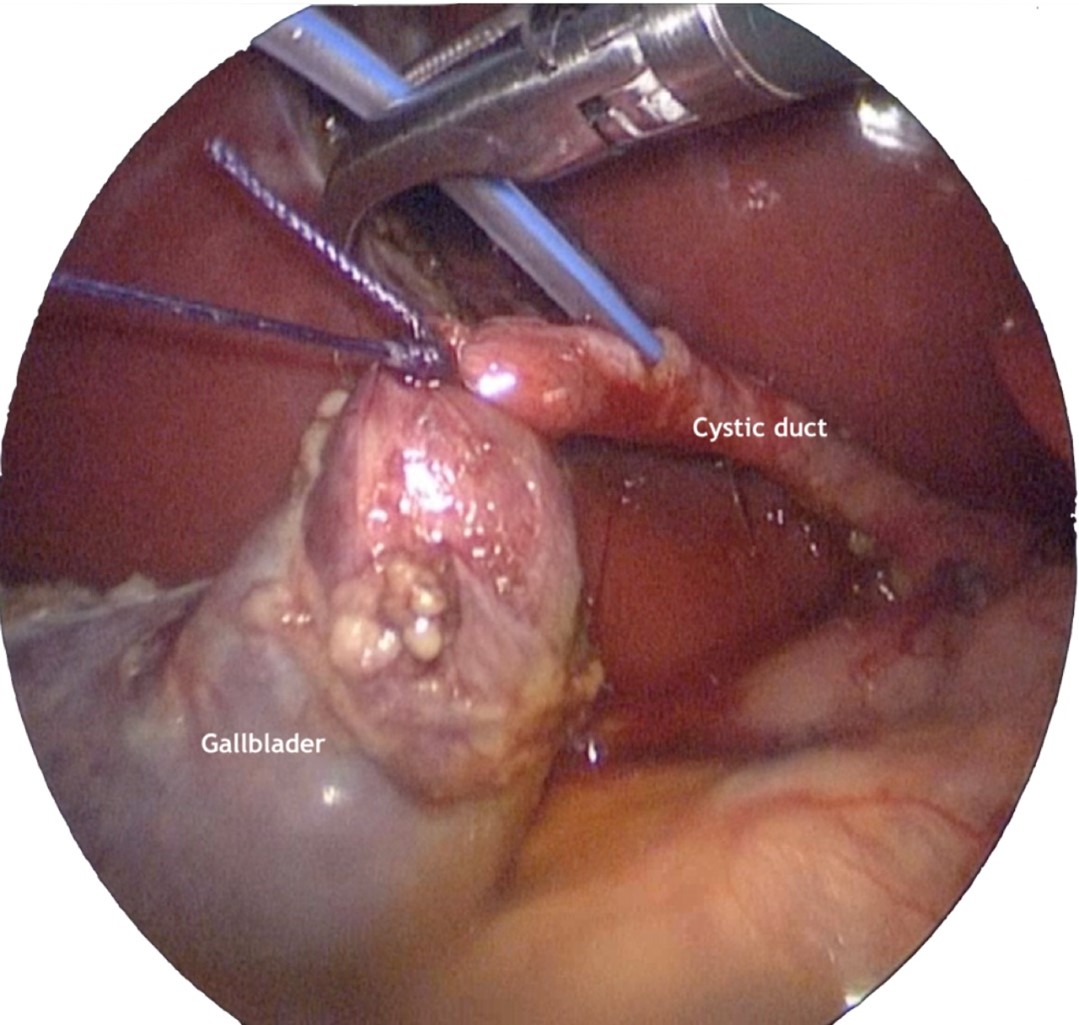
Figure 2: The gallbladder suspended with the endoloop is observed, the transverse incision of the cystic duct and the placement of the 6 Fr. tube for contrast instillation.
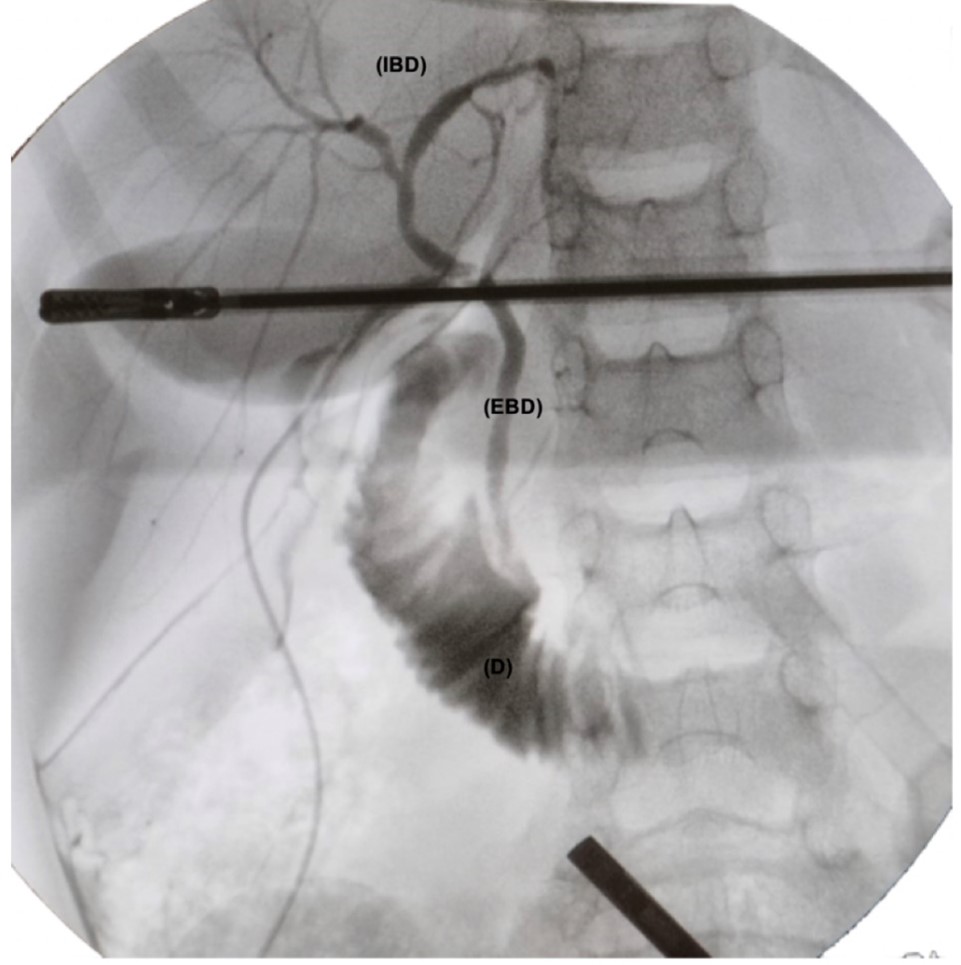
Figure 3: Fluoroscopic image of the intraoperative cholangiography showing the intra (VBI) and extrahepatic (EBV) bile duct and the duodenum (D), without filling defects. The two artifacts are the clamp and the laparoscopy optic.
Discussion
The currently available scientific evidence suggests that the simultaneous performance of laparoscopic splenectomy and cholecystectomy is feasible and safe in paediatric patients with HS [3]. However, expert consensus determines the independent indications for splenectomy and cholecystectomy, so not every patient with HS will benefit from performing both techniques at the same time [2-4].
Splenectomy is indicated in patients with severe anaemia, preferably after 6 years of age given the high risk of post-splenectomy infections due to encapsulated microorganisms [5]. The severity of anaemia is determined by analytical parameters (Table 1) [2-4]. The size of the spleen is not per se an splenectomy indication, nor is there evidence that defends the limitation of physical activity in patients with splenomegaly [1]. Currently, only in cases of concomitant symptomatic cholelithiasis is simultaneous cholecystectomy indicated; while in asymptomatic cholelithiasis there is less evidence in this regard. Liu et al. published the first study concluding that asymptomatic cholelithiasis progresses or persists after splenectomy and therefore simultaneous cholecystectomy reduces long-term complications [6]. And Mack et al. suggest that concurrent cholecystectomy does not increase morbidity and can therefore be performed even in ASA 3 or 4 patients [7]. These publications seem to indicate the beginning of a change in trend towards the simultaneous performance of both procedures; however, more studies are necessary to help corroborate these theories and change clinical practice.
On the other hand, isolated cholecystectomy is indicated in patients with symptomatic cholelithiasis. The indications for simultaneous splenectomy should be the same as those for patients who do not require cholecystectomy [2-4].
Following the previously described indications, the need for concurrent splenectomy was ruled out in our patient given that he had moderate anaemia (haemoglobin 8-12 g/dL, reticulocyte count >10% and total bilirubin >2 mg/dL) without limitation of his usual activity nor transfusions dependency. Furthermore, it was considered that the laparoscopic approach could be hindered by the large size of the spleen, and that performing both techniques could involve a very long surgical time with the consequent increase in morbidity.
In relation to the usefulness of IOC, there is currently no consensus on the need to associate laparoscopic cholecystectomy with routine IOC vs. selective. Kovács et al. published a meta-analysis where they determined that the selective indication of IOC may be appropriate, combining it with measures that prevent CBD injury (critical safety vision, initial approach to the fundus, use of multiple ports, low conversion threshold, etc.) and performing preoperative tests (abdominal, laparoscopic or endoscopic ultrasound or cholangio-MRI) [8].
In paediatrics, risk criteria have been suggested for the diagnosis of choledocholithiasis (CBD dilation >6 mm, choledocholithiasis on ultrasound, or total bilirubin >1.8 mg/dL) [9]. This patient was considered a candidate for CBD exploration because he had persistent elevation of total bilirubin (4.05 mg/dL) at the expense of direct bilirubin (2.23 mg/dL). Given the small size of the cystic duct, it was not possible to proceed with endoscopic exploration of the CBD (only a 5 mm choledochoscope was available) [10]. The IOC ruled out the presence of CBD stones or other CBD anomalies. It is a technique with low risk of complications (bleeding, bile leak, conversion) that allows visualization of the anatomy of the CBD in real time and reduces the postoperative risk of persistent lithiasis, bile leak or iatrogenic CBD injuries [11].
Conclusion
The management of HS represents a therapeutic challenge for the haematologist and paediatric surgeon, who must take into consideration clinical and analytical aspects, as well as the means available in their centre to offer the safest and most appropriate management to each patient.
References
- Boaro MP. Hematological characteristics and hepatobiliary complications of hereditary spherocytosis in a tertiary care pediatric center. FP, 2023; 11: 1269645.
- Bolton‐Maggs PHB. Guidelines for the diagnosis and management of hereditary spherocytosis – 2011 update. BJH, 2012; 156(1): 37-49.
- Schizas D, Katsaros I, Karatza E, Kykalos S, Spartalis E, Tsourouflis G, et al. Concomitant Laparoscopic Splenectomy and Cholecystectomy. JLAST, 2020; 30(7): 730-736.
- Iolascon A. Recommendations regarding splenectomy in hereditary hemolytic anemias. Haematologica, 2017; 102(8): 1304-1313.
- Rothman JA. How I approach hereditary hemolytic anemia and splenectomy. PBC, 2020; 67(11): e28337.
- Liu Y. Treatment of asymptomatic gallstones in children with hereditary spherocytosis requiring splenectomy. JPS, 2023; 58(4): 756-761.
- Mack SJ. Concurrent Cholecystectomy Does Not Increase Splenectomy Morbidity in Patients With Hemolytic Anemia. SJP, 2023; S002234682300547X.
- Kovács N. Selective intraoperative cholangiography should be considered over routine intraoperative cholangiography during cholecystectomy. SE, 2022; 36(10): 7126-7139.
- Ignacio RC. Pediatric DUCT Score. JACS, 2023; 236(5): 961-970.
- Sirimanna P. Laparoscopic common bile duct exploration. AJS, 2023; ans.18756.
- Osailan S. The Use of Intraoperative Cholangiography During Cholecystectomy. CJMS, 2023.

