Uterine Rupture in a Second Trimester Cesarean Scar Pregnancy: A Case of Ultrasound-Guided Suction Aspiration
Patrícia Gomes Ferreira*, Susana Saraiva and Foo Kok Mak
Department of Obstetrics and Gynecology, Centro Hospitalar de Entre Douro e Vouga, Santa Maria da Feira, Portugal
Received Date: 13/10/2024; Published Date: 07/11/2024
*Corresponding author: Patrícia Gomes Ferreira, Department of Obstetrics and Gynecology, Centro Hospitalar de Entre Douro e Vouga, Santa Maria da Feira, Portugal
Abstract
Cesarean Scar Pregnancy (CSP) is an abnormal implantation of the gestational sac in the area of a prior cesarean delivery scar, potentially leading to life-threatening complications. While diagnosis has improved with advancements in ultrasonography, the optimal management of CSP remains unclear, and no standard of care has been established. While treatment options have been documented successfully in early pregnancies, they have not been extensively explored in pregnancies diagnosed at later gestational ages.
We present a challenging case of CSP diagnosed in the second trimester and treated with ultrasound-guided suction aspiration followed by silicone balloon insertion.
Keywords: Cesarean scar pregnancy; Uterine rupture; Second trimester
Introduction
A Cesarean Scar Pregnancy (CSP) occurs when the gestational sac implants "on the scar" or "in the niche" after a previous cesarean section [1]. "On the scar" CSP, the gestational sac is implanted on the well-healed scar [2], with a measurable myometrial thickness between the placenta/gestational sac and the anterior uterine surface or the bladder [1,3]. In contrast, "in the niche" CSP involves a deeply implanted gestational sac into a deficient or dehiscent scar [2].
CSP is associated with a wide range of adverse short- and long-term outcomes, including massive hemorrhage, uterine rupture, the need for hysterectomy, and placenta accreta spectrum (PAS) disorders [4]. The incidence of CSP is estimated to be between 0,05% and 0,4% of all pregnancies and is expected to rise as cesarean section rates increase. The pathophysiology of CSP is not fully understood. One possible mechanism is that trauma caused by a cesarean section creates microscopic tracts through which an implanting blastocyst abnormally invades the affected myometrium. CSP is typically diagnosed through transvaginal ultrasound (TVUS) findings [5]. The gestational age at the time of ultrasound also significantly impacts the detection rate of CSP. Prenatal diagnosis of CSP is more easily achieved in the early first trimester of pregnancy (<9 weeks gestation). As gestation advances, the upper pole of the gestational sac grows toward the uterine fundus, making prenatal identification of CSP more challenging [6]. Failure to identify this condition in a timely manner could lead to increased maternal morbidity and mortality [7].
A variety of therapeutic strategies have been described, either as standalone treatments or as part of combined management plans. The most frequently reported interventions include systemic or local injection of methotrexate into the gestational sac, uterine artery embolization, suction curettage, laparoscopic or laparotomic resection, and compression using a single or double balloon catheter [8]. However, the optimal approach in terms of outcomes and patient safety has yet to be determined.
We present a case of second-trimester CSP complicated by uterine rupture following management.
Case Presentation
A 27-year-old woman, a smoker, with a history of a cesarean section 11 years ago and a subsequent vaginal delivery, underwent excision of the transformation zone for cervical intraepithelial neoplasia grade 2 about two years ago. She presented to the emergency department with moderate vaginal bleeding and mild pelvic discomfort, with a 14-week history of amenorrhea.
On admission, her vital signs indicated slight tachycardia. Abdominal examination revealed a soft abdomen with mild tenderness. Vaginal examination showed a closed cervix with minimal vaginal bleeding, and bimanual palpation revealed tenderness at the anterior fornix. Transvaginal Ultrasound (TVUS) confirmed the presence of a gestational sac containing a live fetus, with the placenta implanted at the site of the previous cesarean scar. The myometrial thickness between the gestational sac and the bladder wall was 3 mm. The biparietal diameter corresponded to 15 weeks and 6 days of gestation, with the presence of cardiac activity. There were no signs of placental abruption (Figure 1).
This was an unplanned pregnancy, and the risks of continuing the pregnancy were explained to the patient. She opted for pregnancy termination. Ultrasound-guided suction aspiration was proposed and accepted by the patient. The procedure was performed under general anesthesia, and a silicone balloon was inserted into the lower uterine segment, filled with 60 ml of normal saline.
Two hours post-surgery, the patient became hemodynamically unstable, showing signs of hypovolemic shock. Immediate preparations were made for an emergency laparotomy, along with fluid and blood resuscitation (two units of red blood cells). Uterine rupture with significant hemoperitoneum was suspected. A midline sub-umbilical laparotomy was performed, revealing a retroperitoneal hematoma near the right ovary without active bleeding, extending to the lower segment of the anterior uterine wall. Abdominal packing was conducted in the pelvic cavity and right flank. Antibiotic prophylaxis was initiated, and the patient was transferred to the intensive care unit.
On the first postoperative day, the patient was hemodynamically stable, with a hemoglobin level of 8,7 g/dL. However, by the second day, her hemoglobin had decreased to 7,5 g/dL. Bilateral arterial embolization was considered, but a Computed Tomography (CT) angiography showed no signs of active bleeding. On the third day, the patient remained hemodynamically stable, and an abdominopelvic CT scan revealed an oval, hyperdense lesion measuring 70 × 50 mm on the right side of the uterus, suggestive of a hematoma with no signs of active bleeding. The abdominal packing was removed, and surgical drains were placed on the same day. On the ninth postoperative day, a TVUS showed the endometrium appeared poorly defined, measuring 7 mm in thickness. The retroperitoneal hematoma measured 71 × 30 mm (Figure 2). The abdominal drains were removed, and the patient was discharged.
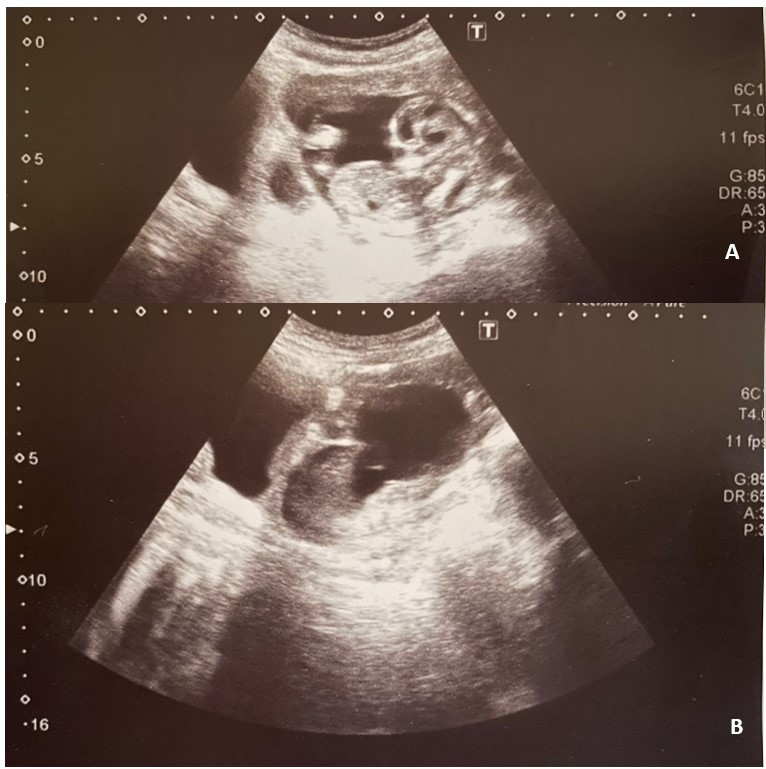
Figure 1: Sagittal transvaginal ultrasounds demonstrating cesarean scar pregnancy located in closed proximity to bladder. A. Gestational sac with fetus with cardiac activity. B. Myometrial thickness of 3 mm between the placenta and bladder.
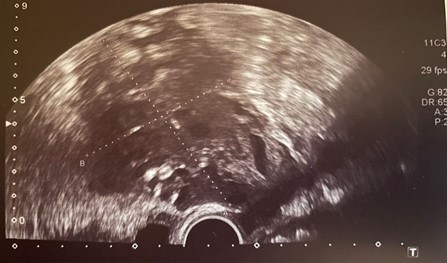
Figure 2. Retroperitoneal hematoma.
Discussion
This clinical case is challenging due to a late diagnosis of CSP, presenting in the emergency department with abdominal pain and vaginal bleeding.
The expectant management is associated to high morbidity. In a meta-analysis looking at 17 different studies of expectant management in patients with CSP resulted in high burden of maternal morbidity including severe hemorrhage, early uterine rupture, and hysterectomy [9]. “PAS” has been reported in over 50% of CSP cases across various case series, likely reflecting differing diagnostic criteria in the published literature [9,2]. Given the severe maternal morbidity and mortality associated with a CSP, the Society for Maternal-Fetal Medicine, the American College of Obstetricians and Gynecologists and the American Society of Reproductive Medicine, recommends against expectant management of CSP [3]. The most usual recommendation has been termination of pregnancy [9,3], with a multitude of medical and surgical managements.
Given the lack of randomized controlled trials comparing the different treatment options, there is not one recommended management strategy [3]. The choice of treatment often depends on the specialized skills available within an institution, which may include a minimally invasive gynecologic surgeon, a maternal-fetal medicine specialist, or an interventional radiologist.
For medical management of a CSP, intragestational sac injection of methotrexate with or without systemic methotrexate has high rates of success with low rates of complications, but the patient should be counseled to expect prolonged follow-up [10].
Sharp curettage has the potential to expose deeply invasive blood vessels, leading to hemorrhage, and consequently should be avoided. However, vacuum aspiration has been shown in multiple studies to be efficacious with low rates of complications. Suction aspiration is a commonly used surgical treatment for CSP [11]. It is typically performed for patients in the early first trimester (five to seven weeks of gestation) and can be combined with transcervical balloon catheters [12,13].
In one prospective study including eight cases of CSP (gestational age between 4 to 23 weeks of gestation) treated with suction curettage, all cases were treated successfully, however, three patients (37%) experienced significant bleeding (500 to 1000 mL) requiring the insertion of an intrauterine balloon catheter to tamponade the uterine cavity to achieve hemostasis [12].
Operative resection of the pregnancy can be performed via laparoscopy, hysteroscopy, or laparotomy; laparoscopic-assisted operative hysteroscopic management has also been described [14]. Surgical resection may also be performed as subsequent management after medical therapy (for example, intragestational injection of methotrexate). These procedures must be performed by an experienced surgical team. An advantage of resection over other therapies is that the scar can be excised and the uterus reapproximated.
Laparoscopic excision had a success rate of 97,1% without complications in one systematic review that included 69 cases [8]. In 118 cases of transvaginal excision, there was > 99% efficacy and a 0,9% complication rate [8]. Hysteroscopic excision of a CSP has an efficacy of 83% and a complication rate of 3,2% in 95 cases [15]. Laparotomy had a complication rate of 5,3% [8]. These options are more invasive than those described above and may be more suitable for more advanced gestations.
Hysterectomy should be performed in patients in whom future childbearing is not desired or in those with life-threatening hemorrhage. Gravid hysterectomy may be performed with or without prior uterine artery embolization to decrease the amount of blood loss [16].
The less invasive surgical intervention was chosen, with ultrasound-guided aspiration followed by balloon insertion. However, in this case, a known and possible complication occurred: hemorrhage caused by uterine rupture. In this case, it was possible to control the hemorrhage with pelvic packing, preserving the uterus.
Conclusion
This case highlights the importance of considering CSP in the differential diagnosis for any pregnant patient with a history of cesarean section who presents with vaginal bleeding or shows concerning signs on ultrasound, such as a low-lying embryo. Additionally, women should be counseled after their primary cesarean section about the rare but increased risk of CSP due to prior uterine surgery, and they should seek evaluation promptly if they experience vaginal bleeding early in a subsequent pregnancy.
Proper diagnosis and differentiation of CSP types are critical, as they provide vital information for comprehensive counseling. This allows patients to make informed decisions about whether they are willing to face the significant health risks involved in continuing the pregnancy to potentially deliver a live infant [10].
In general, management guidelines for CSP are limited, particularly for cases diagnosed in the second trimester. Many conservative treatment modalities are likely only effective when CSP is identified early in gestation. Our case underscores the importance of early diagnosis to expand treatment options. In this instance, we adopted a minimally invasive approach to reduce the patient’s risk as much as possible. Although a less invasive surgical option was selected in our case, a severe complication still occurred. Further research and clearer guidelines are necessary for this rare condition, which is expected to become more prevalent due to the increasing rates of cesarean deliveries.
Consent for Publication: Written informed consent was obtained from the patient for publication of this case report and accompanying images.
References
- Timor-Tritsch IE, Monteagudo A, Cali G, D’Antonio F, Kaelin Agten A. Cesarean Scar Pregnancy: Diagnosis and Pathogenesis. Obstet Gynecol Clin North Am, 2019; 46: 797–811.
- Kaelin Agten A, Cali G, Monteagudo A, Oviedo J, Ramos J, Timor-Tritsch I. The clinical outcome of cesarean scar pregnancies implanted "on the scar" versus "in the niche". American journal of obstetrics and gynecology, 2017; 216(5): 510.e1–510.e6. https://doi.org/10.1016/j.ajog.2017.01.019.
- Miller R, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy. Am J Obstet Gynecol, 2022; 227(3): B9–b20.
- D'Antonio F, Cali G, Khalil A, Timor-Tritsch T: Cesarean Scar Pregnancy, Visual Encyclopedia of Ultrasound in Obstetrics and Gynecology, www.isuog.org, 2022.
- Odgers HL, Taylor RAM, Balendran J, Benness C, Ludlow J. Rupture of a caesarean scar ectopic pregnancy: A case report. Case Rep Womens Health, 2019; p. e00120.
- Timor-Tritsch IE, Monteagudo A, Cali G, Vintzileos A, Viscarello R, Al-Khan A, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology, 2014; 44(3); 346–353. doi: https://doi.org/10.1002/uog.13426.
- Ajong AB, Kenfack B, Agbor VN, Njotang PN. Ruptured caesarean scar ectopic pregnancy: a diagnostic dilemma in a resource-limited setting. BMC Res Notes, 2018; 11(1): 292.
- Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril, 2016; 105(4): 958-967. doi: 10.1016/j.fertnstert.2015.12.130.
- Calì G, Timor-Tritsch IE, Palacios-Jaraquemada J, et al. Outcome of Cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol, 2018; 51: 169.
- Lin R, DiCenzo N, Rosen T. Cesarean scar ectopic pregnancy: nuances in diagnosis and treatment. Fertility and sterility, 2023; 120(3 Pt 2): 563–572. https://doi.org/10.1016/j.fertnstert.2023.07.018.
- Qian ZD, Weng Y, Du YJ, et al. Management of persistent caesarean scar pregnancy after curettage treatment failure. BMC Pregnancy Childbirth, 2017; 17: 208.
- Jurkovic D, Hillaby K, Woelfer B, et al. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol, 2003; 21: 220.
- Wu C, Li Y, Ye W, et al. Cook Cervical Ripening Balloon successfully prevents excessive hemorrhage combined with ultrasound-guided suction curettage in the treatment of cesarean scar pregnancy. J Obstet Gynaecol Res, 2017; 43: 1043.
- Robinson JK, Dayal MB, Gindoff P, Frankfurter D. A novel surgical treatment for cesarean scar pregnancy: laparoscopically assisted operative hysteroscopy. Fertil Steril, 2009; 92: 1497.e13.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012; 207(1): 14–29. https:// doi.org/10.1016/j.ajog.2012.03.007.
- Liu S, Durai S. Management of a case of caesarean scar pregnancy and all its complications. BMJ Case Rep, 2016; 2016.

