Fulminant Type 1 Diabetes in an Elderly Patient: A Case Report from a Community Hospital
Xing Yan Choo1, Chiang Wen Teo1, Su-Fee Lim2 and Luke Sher Guan Low2
1Department of Post-Acute and Continuing Care, Outram Community Hospital, Singapore
2SingHealth Community Hospitals, Singapore
Received Date: 21/08/2024; Published Date: 24/10/2024
*Corresponding author: Xing Yan Choo, Department of Post-Acute and Continuing Care, Outram Community Hospital, Outram Community Hospital, 10 Hospital Blvd, Singapore 168582
Abstract
This case report describes a 70-year-old male with a history of atypical Parkinson's disease who developed severe metabolic disturbances and was diagnosed with Fulminant Type 1 Diabetes Mellitus (FT1DM) following an episode of Diabetic Ketoacidosis (DKA). FT1DM is characterized by the rapid and near-total destruction of pancreatic β-cells, leading to severe insulin deficiency and the abrupt onset of DKA. Unlike classical Type 1 diabetes, FT1DM presents suddenly and is often not associated with typical islet-related autoantibodies. Initial evaluation revealed critical metabolic imbalances and severe insulin deficiency, with negative autoimmune markers. The patient was treated with immediate intravenous insulin and aggressive fluid resuscitation, followed by long-term insulin therapy and regular monitoring. This case underscores the importance of recognizing FT1DM in patients without a prior history of diabetes, emphasizing the need for early diagnosis and prompt management to improve outcomes in this rapidly progressing and life-threatening condition.
Keywords: Fulminant Type 1 Diabetes Mellitus; Undiagnosed diabetes; Type 1 Diabetes Mellitus; Hyperglycemia; Diabetic Ketoacidosis
Introduction
Fulminant Type 1 Diabetes Mellitus (FT1DM) is a recently recognized and aggressive form of diabetes, characterized by the abrupt and near-total destruction of pancreatic β-cells, leading to severe insulin deficiency. [1]. Unlike classical Type 1 diabetes, FT1DM manifests with a rapid onset of Diabetic Ketoacidosis (DKA), often within days of initial hyperglycemic symptoms. This condition is notable for its unique clinical presentation, where patients exhibit high plasma glucose levels juxtaposed with near-normal glycated hemoglobin (HbA1c) levels, reflecting the disease's brief duration before diagnosis. The etiology of FT1DM remains elusive but has been associated with various triggers, including viral infections and immune responses that precipitate β-cell destruction [2]. This case report discusses a 70-year-old male with atypical Parkinson's disease, who presented with non-specific gastrointestinal symptoms and was ultimately diagnosed with FT1DM following an episode of DKA. This case underscores the need for heightened clinical awareness of FT1DM, especially in patients with no prior history of diabetes, to ensure timely diagnosis and management of this life-threatening condition.
Case Presentation
A 70-year-old male with a history of atypical Parkinson's disease, treated with Madopar 250 mg four times daily, was admitted to a tertiary hospital after an unwitnessed fall resulting in an L2 compression fracture. After stabilization, he was transferred to a community hospital for rehabilitation. He had no prior diagnosis of diabetes; his HbA1c one year ago was 5.6%, and fasting plasma glucose levels were normal during this admission.
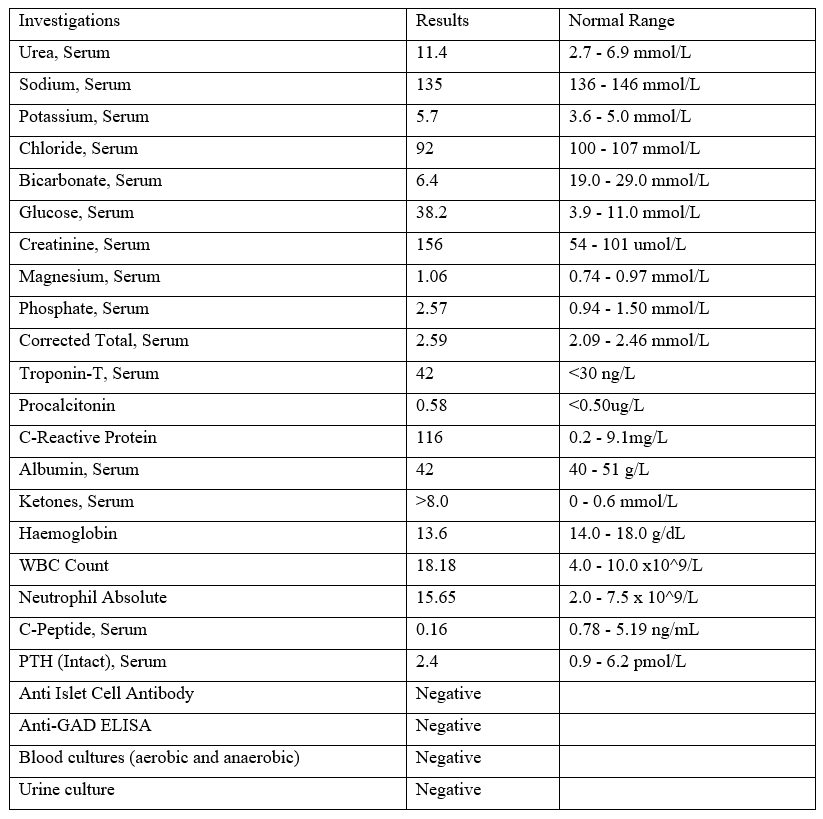
After a month at the community hospital, he experienced three episodes of non-bilious, non-bloody vomiting, managed with domperidone. That evening, he reported non-specific central chest pain with ST depression observed in leads V2-V3 on the ECG. The patient appeared clinically dehydrated and exhibited tachycardia, with a heart rate of 100-110 bpm. Laboratory tests (refer to Table 1) revealed severe metabolic acidosis, a serum bicarbonate level of 6.4 mmol/L, a pH of 7.08, a random plasma glucose level of 38.2 mmol/L, and serum ketones greater than 8 mmol/L. Remarkably, his HbA1c was only 6.6% despite the high plasma glucose level. Troponin T was slightly raised at 42 ng/L, and creatinine at 156 µmol/L indicated acute cardiac and kidney injury.
He was diagnosed with DKA and transferred back to the acute hospital for further management. Intravenous insulin and aggressive fluid resuscitation were administered. A concurrent diagnosis of Type 2 Myocardial Infarction (T2MI) was made, necessitating the initiation of dual antiplatelet therapy. Further history revealed no osmotic symptoms before the onset, no family history of diabetes, and no medications that could induce hyperglycaemia. His BMI was 21.1kg/m2, and there was no evidence of acanthosis nigricans. The patient recovered from his DKA and responded well to subcutaneous insulin during his stay in the general medical ward. Upon transfer back to the community hospital for continued rehabilitation, the patient opted for oral anti-diabetic medications, refusing long-term insulin. He was prescribed Metformin 500mg twice daily, Glipizide 5mg before breakfast, and Glipizide 2.5mg before dinner.
A few days after his return to the community hospital, the patient’s capillary glucose levels became unstable, and he developed ketosis again. He was immediately switched back to basal-bolus insulin therapy. The endocrinology team conducted further investigations, revealing a post-prandial serum C-peptide of only 0.16 ng/mL despite a high plasma glucose level of 10 mmol/L, indicating severe insulin deficiency. A CT abdomen and pelvis showed no pancreatic mass or swelling to suggest pancreatitis. Thyroid and parathyroid panels were unremarkable. Islet-related autoimmune markers such as anti-GAD and anti-islet cell antibodies were negative. Given the rapid onset of DKA, the absence of a previous history of diabetes, near-normal HbA1C at onset, and severe insulinopenia, a diagnosis of FT1DM was made based on the Japan Diabetes Society 2012 criteria.
During his stay in the community hospital, a comprehensive multidisciplinary plan was implemented for the long-term management of FT1DM. A person-centered team care approach and long-term treatment approaches were adopted, with close collaboration and goal-setting among family physicians, endocrinologists, nurses, dietitians, physical therapists, and a medical social worker. In addition to medical therapy, the patient received extensive education on blood sugar control, medication compliance, dietary and exercise plans, insulin administration, blood glucose monitoring, lifestyle modifications, and access to online and community resources. Psychosocial support, including counseling services, was provided to help the patient and his family cope with the diagnosis. Social support networks were established to support the patient in the community. This multidisciplinary approach underscores the importance of coordinated care in managing FT1DM, addressing both physical and emotional health. He was discharged with a basal-bolus insulin regime and scheduled for follow-up with endocrinology.
Table 1: Laboratory Investigations.
Discussion
Type 1 diabetes (T1DM) is characterised by absolute insulin deficiency due to the destruction of pancreatic β-cells. It is classified into autoimmune (type 1A) and idiopathic (type 1B) forms. Most T1DM cases are immune-mediated, indicated by the presence of autoantibodies against islet-related antigens such as glutamic acid decarboxylase (GAD), insulin, the tyrosine phosphatases islet antigen 2 (IA-2), and zinc transporter 8 (ZnT8). Up to 15% of individuals lack these autoantibodies and are termed idiopathic, with their β-cell destruction mechanisms remaining unclear [3].
Fulminant type 1 diabetes (FT1DM), first described by Imagawa et al. in 2000, is a distinct subtype of T1DM characterised by the rapid, nearly total destruction of pancreatic β-cells [4]. This leads to acute insulinopenia, severe ketoacidosis, and potential death without immediate treatment. The disease progresses from a normoglycemic state to β-cell destruction and ketoacidosis within days, rarely exceeding a week [5]. This rapid progression contrasts with classic autoimmune T1DM (AT1DM), which develops more slowly, over weeks to months, with gradual metabolic derangement often accelerated towards DKA by acute triggers like infections and cardiac events. Unlike FT1DM, A1TDM has a longer prodromal period with osmostic symptoms, high HBA1c levels (reflecting a longer duration of absolute insulin deficiency and hyperglycaemia) and positive islet-related autoantibodies [6].
While AT1DM is more prevalent in Caucasians, FT1DM has a higher incidence in Asian populations [7]. The exact mechanisms of β-cells destruction are unknown but likely involve genetic factors and immune responses to viral infections and immune checkpoint inhibitors used in cancer treatment [2]. The prevalence of FT1DM is about 14-20% of ketosis-onset T1DM cases [8,9]. In a case series by Tan et al., seven individuals were identified over 8 years from two tertiary centers in Singapore [10]. More than 90% of FT1DM cases occurred in adults, with an older age of onset compared to AT1DM. No significant sex difference was observed among cases. Both groups of patients generally have a lean BMI and do not report a significant family history of diabetes mellitus, although other autoimmune diseases are more frequently observed in AT1DM than in FT1DM [8].
The Japan Diabetes Society's 2012 criteria diagnose FT1DM if all the following characteristics are fulfilled [11]:
- Diabetic ketosis or ketoacidosis occurs soon (approximately 7 days) after the onset of hyperglycemia symptoms.
- Plasma glucose ≥16.0 mmol/L (≥288 mg/dL) and HbA1c <8.7% at first visit.
- Urinary C-peptide excretion <10 mg/day or fasting serum C-peptide <0.3 ng/mL (<0.10 nmol/L) and <0.5 ng/mL (<0.17 nmol/L) after intravenous glucagon (or after meal) load at onset.
Other supportive findings include generally undetectable islet-related autoantibodies; short duration of disease before the start of insulin treatment; elevation of serum pancreatic enzymes; flu-like symptoms (fever, upper respiratory symptoms) or gastrointestinal symptoms (upper abdominal pain, nausea, and/or vomiting); occurrence during pregnancy or just after delivery; and association with HLA DRB104:05-DQB104:01 [11]. In this case report, our patient developed DKA after one day of vomiting symptoms and met all three diagnostic criteria for FT1DM. Autoantibodies were negative and he became insulin-dependent shortly after the disease onset.
Immediate treatment is critical upon FT1DM diagnosis to prevent rapid deterioration and death. The treatment involves prompt intravenous infusion of saline and regular insulin for managing ketoacidosis, similar to AT1DM management. Failure to diagnose FT1DM can be fatal [5]. Unlike AT1DM, where pancreatic β-cell function declines gradually, FT1DM patients experience immediate and complete insulin deficiency, leading to high glycaemic variability, frequent severe hypoglycemia, and higher total daily insulin requirements [11]. After managing the acute phase of ketoacidosis, patients usually require multiple daily insulin injections and sometimes early use of continuous subcutaneous insulin infusion therapy with a portable pump [5]. FT1DM patients also face a higher incidence of microvascular complications within the first five years of diagnosis due to unstable blood glucose control [12]. High-quality diabetes self-management and support have been shown to improve self-management, satisfaction, and glycemic outcomes. Comprehensive patient education on diabetes management and psychosocial support is essential to help patients and their families cope with the chronic nature of the disease. Additionally, increased social support is associated with better glycemic control and improved quality of life while lack of social support has been associated with increased mortality and diabetes related complications for individuals with diabetes [11,12].
Conclusion
FT1DM is a rapidly progressing, life-threatening, yet often underrecognized subtype of type 1 diabetes that requires immediate treatment. It frequently develops in seemingly healthy individuals without a prior diabetes diagnosis, and its prodromic infection history may mislead physicians to treat it as an upper respiratory tract infection, acute gastroenteritis, or pancreatitis [6]. Increasing awareness among physicians about FT1DM is crucial to improve early recognition and management.
Author’s Contribution: Xing Yan and Chiang Wen has contributed to the drafting of the case report. Su-Fee and Luke Sher Guan reviewed and revised the manuscript. All authors were involved in the final revision of the manuscript and approved its submission.
Conflict of Interest Statements: The authors of this case report, declare that we have no conflicts of interest related to the content of this manuscript.
Funding Source Declaration: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Ata F, Khan AA, Khamees I, AlKodmani S, Al-Sadi A, Yaseen KB, et al. Prevalence and prognosis of fulminant type 1 diabetes mellitus in The Middle East: A com-parative analysis in a 5-year nationwide cohort. BMC Endocrine Disorders, 2024; 24(1): 33. https://doi.org/10.1186/s12902-024-01559-8.
- Hosokawa Y, Hanafusa T, Imagawa A. Pathogenesis of fulminant type 1 diabetes: Genes, viruses and the immune mechanism, and usefulness of patient‐derived induced pluripotent stem cells for future research. Journal of Diabetes Investigation, 2019; 10(5): 1158–1164. https://doi.org/10.1111/jdi.13091.
- American Diabetes Association Professional Practice Committee, El Sayed NA, Aleppo G, Bannuru RR, Bruemmer D, Collins BS, et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes, 2024; Diabetes Care, 47(Supplement_1): S20–S42. https://doi.org/10.2337/dc24-S002.
- Imagawa Akihisa, Hanafusa Toshiaki, Miyagawa Jun-ichiro, Matsuzawa Yuji. A Novel Subtype of Type 1 Diabetes Mellitus Characterized by a Rapid Onset and an Absence of Diabetes-Related Antibodies. New England Journal of Medicine, 2000; 342(5): 301–307. https://doi.org/10.1056/NEJM200002033420501.
- Hanafusa T, Imagawa A. Fulminant type 1 diabetes: A novel clinical entity requiring special attention by all medical practitioners. Nature Clinical Practice Endocrinology & Metabolism, 2007; 3(1): 36–45. https://doi.org/10.1038/ncpendmet0351.
- Lane AS, Champion B, Orde S, Dravec D. Diabetic ketoacidosis due to fulminant type 1 diabetes: A rare subtype of type 1 diabetes leading to unusual sequelae. Journal of the Intensive Care Society, 2015; 16(1): 64–70. https://doi.org/10.1177/1751143714551249.
- You W, Yang J, Liu Y, Wang W, Zhu L, Wang W, et al. Fulminant type 1 diabetes mellitus. Medicine, 2019; 98(5): e14319. https://doi.org/10.1097/MD.0000000000014319.
- Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, et al. Fulminant Type 1 Diabetes. Diabetes Care, 2003; 26(8): 2345–2352. https://doi.org/10.2337/diacare.26.8.2345.
- Sano H, Imagawa A. Re-Enlightenment of Fulminant Type 1 Diabetes under the COVID-19 Pandemic. Biology, 2022; 11(11): Article 11. https://doi.org/10.3390/biology11111662.
- Tan SYT, Rama Chandran S, Yew J, Wong AJ, Gardner DS. Fulminant type 1 diabetes, an un-derrecognized and unique subtype of type 1 diabetes: A case series from Singapore. Journal of Diabetes Investiga-tion, 2024; 15(6): 786–789. https://doi.org/10.1111/jdi.14160.
- Imagawa A, Hanafusa T, Awata T, Ikegami H, Uchigata Y, Osawa H, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute‐onset Type 1 Diabetes Mellitus: New diagnostic criteria of fulminant type 1 diabetes mellitus. Journal of Diabetes Investigation, 2012; 3(6): 536–539. https://doi.org/10.1111/jdi.12024.
- American Diabetes Association Professional Practice Committee (2023b). Summary of Revisions: Standards of Care in Diabetes, 2024. Diabetes Care, 47(Supplement_1): S5–S10. https://doi.org/10.2337/dc24-SREV
- Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care, 2020; 44: 258–279.
- Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, et al. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care, 2020; 44(1): 258–279. https://doi.org/10.2337/dci20-0053
- Murase Y, Imagawa A, Hanafusa T, Iwahashi H, Uchigata Y, Kanatsuka A, et al. Fulminant type 1 diabetes as a high risk group for diabetic microangiopathy—A nationwide 5-year-study in Japan. Diabetologia, 2007; 50(3): 531–537. https://doi.org/10.1007/s00125-006-0575-y.
- Patel SK, Ma CS, Fourlanos S, Greenfield JR. Autoantibody-Negative Type 1 Diabetes: A Ne-glected Subtype. Trends in Endocrinology & Metabolism, 2021; 32(5): 295–305. https://doi.org/10.1016/j.tem.2021.02.001.

