The Case of Deep Vein Thrombosis Complicated by Massive Pulmonary Embolism in the setting of Disseminated Intravascular Coagulopathy: What Can We Do?
Ai Xin Lee and Si Ching LIM*
Department of Geriatric Medicine, Changi General Hospital, Singapore
Received Date: 29/07/2024; Published Date: 17/10/2024
*Corresponding author: Si Ching Lim, Adjunct Associate Professor, Senior Consultant, Department of Geriatric Medicine, Changi General Hospital, Singapore
Abstract
Disseminated Vascular Coagulation (DIC) is a consumptive coagulopathy resulting in massive systemic thrombosis and bleeding risks happening at the same time. The case report illustrates an elderly patient who presented with sepsis complicated by DIC, with concomitant Deep Vein Thrombosis (DVT) of bilateral lower limbs which was further complicated by life threatening saddle Pulmonary Embolus (PE). The clinicians faced the challenges of managing venous thromboembolic events with high bleeding risks with anticoagulant, where catheter directed mechanical thrombectomy maybe the only lifesaving option.
Keywords: Pulmonary Embolism; Deep Vein Thrombosis (DVT); Disseminated Intravascular Coagulation (DIC); Thrombectomy, Thrombolysis
Case Report
Madam JA was a 70-year-old lady with a past medical history of hypertension, ischemic heart disease, Left MCA, PCA stroke, and vascular dementia. She presented with a one-week history of poor oral intake, delusional ideation and reduced alertness. Physical examination was unremarkable except for low-grade pyrexia (Temperature 37.6°C). Initial investigations showed Haemoglobin 10.5 g/dL (normal 11.5-15.0 g/dL), platelet count 51 x 10³/μL (normal 150-450 x 10³/μL). White cell count was 7.8 x 10³/μL (normal 4.0-10.0 x 10³/μL). Inflammatory markers were mildly elevated: C-reactive Protein 34 mg/L (normal <3.0 mg/L) which rose to 89.6 mg/L on day 3 and Procalcitonin 1.03 μg/L (normal 0-0.5 μg/L). Significant Acute Kidney Injury (AKI) was noted with raised creatinine levels of 251 μmol/L (normal 50-90 μmol/L). A Computed Tomography Thorax, Abdomen, and Pelvis scan was performed to identify the source of sepsis, which showed bilateral pyelonephritis with right-sided hydronephrosis. There were no evidence of Pulmonary Embolism (PE) reported. She was started on Intravenous (IV) hydration and IV Augmentin and had an indwelling catheter inserted for strict intake/output monitoring. Renal function improved with IV hydration. During her stay in the general ward, her bicytopenia worsened, with a drop of Haemoglobin to 9.7 g/dL and Platelet count of 39 x 10³/μL.
On Day 2 of admission, she was drowsy, opened eyes spontaneously with no meaningful verbal output. There were no other physical findings apart from bilateral calf swelling, with Right calf circumference of 32 cm compared to the left at 28 cm. Her lower extremities were pale, warm and both feet had a mottled branched reticular appearance. Repeat blood investigations showed a further drop in Haemoglobin to 8.9 g/dL, from 9.7g/dl, and Platelet count of 15 x 10³/μL from 39x10³/μL.
Screen for disseminated intravascular coagulopathy (DIC) was requested and showed a prolonged prothrombin time (PT) of 11.8 seconds (normal 9.5-11.5 seconds), Activated Partial Thromboplastin Time (APTT) of 74.8 seconds (normal 24.9-34.0 seconds), reduced fibrinogen level of 1.73 g/L (normal 1.80-4.80 g/L), D-Dimer was elevated at 33.15 (0.19-0.55mg/l FEU), Haptoglobin 386 (30-200mg/dl). Her serum lactate was 5 (normal 0.5-2.2mmol/l), LDH 584 (normal 90-190 U/L). Peripheral blood film showed normochromic normocytic red blood cells, elliptocytes, schistocytes, and occasional target cells. Platelet transfusion was given in view of the extremely low count. Liver function was normal, and IV vitamin K was given. She was taking Aspirin 100mg and Dipyridamole 150mg tds for recurrent strokes and these were promptly discontinued.
Doppler ultrasound of the lower limbs on Day 3 was positive for bilateral deep vein thrombosis (DVT) shown on Figure 1. In view of severe thrombocytopenia and Hb drop, anticoagulation was not given. Due to the extensive bilateral DVT and contraindication for anticoagulant therapy, risks of embolism was conveyed to the family and the option of mechanical thrombectomy and IVC filter were options for consideration.
While the team was preparing for mechanical thrombectomy scheduled on day 4 post admission, she developed tachycardia (heart rate 110 bpm) and oxygen dropped to 85% on room air. She was previously not in respiratory distress and had not been on supplemental oxygen, maintain her oxygen saturation of 93-99% on room air. She was started on 3 L of oxygen via nasal prongs, oxygen saturation improved to 95%. Electrocardiogram (ECG) showed sinus tachycardia and S1Q3T3 pattern, Figure 2. A Computed Tomography Pulmonary Angiogram (CTPA), Figure 3, showed a large filling defect straddling across the pulmonary trunk bifurcation into the left and right main pulmonary arteries with right heart strain. Pulmonary emboli were also seen in both lungs. The intervention radiologist updated and urgent Pulmonary Embolism (PE) thrombectomy was planned. Unfortunately, her condition deteriorated rapidly. She required oxygen with a Non-Rebreather Mask and was hypotensive (systolic blood pressure 80s). Madam JA was not in a fit state to make decision for her treatment plans. Her family was consulted and they wished to keep her comfortable, as was previously agreed by Madam JA for comfort care at her end of life with no heroic or life-sustaining interventions in the event of a life-threatening illness. Decision was therefore made for conservative management. She subsequently passed away 8 hours after the diagnosis of saddle PE.
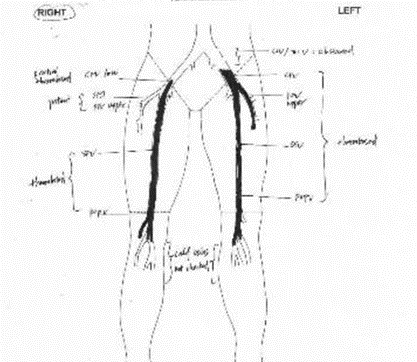
Figure 1: Diagrammatic presentation of bilateral DVT.
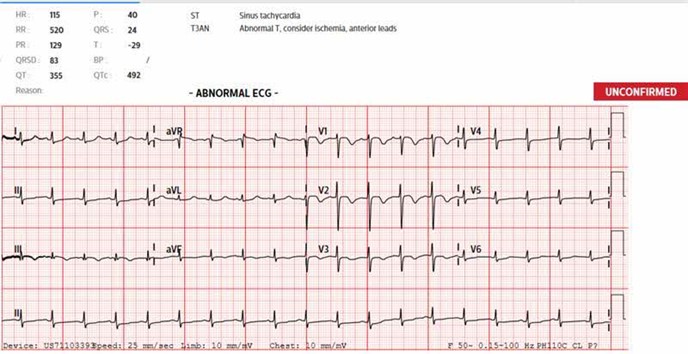
Figure 2: ECG showing sinus tachycardia and S1Q3T3 pattern (classical features of PE).
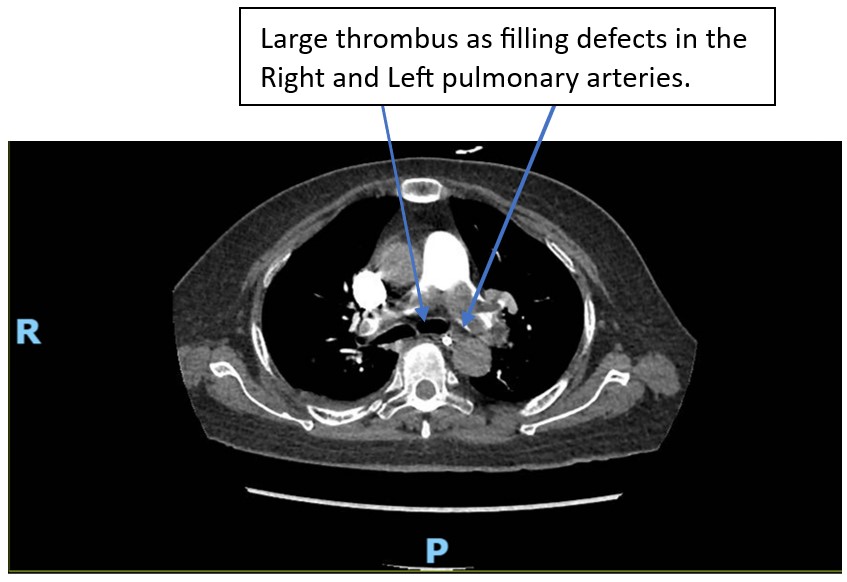
Figure 3: CTPA showing Saddle PE.
Discussion
Disseminated intravascular coagulation (DIC) is a systemic disorder characterized by hypercoagulability leading to micro and macrovascular clotting and hypoperfusion, resulting in multi-organ dysfunction. Normally, when vascular injury occurs, haemostasis ensures clot formation occur rapidly to prevent excessive blood loss. Resolution of clot is necessary to allow blood flow to resume, such that tissue repair can occur. This process is carefully orchestrated in stages which begins with formation of platelet plug, followed by activation of coagulation cascade which ends with a clot formation. When the clot is firmly formed, coagulation cascade ends and fibrinolysis ensues to dissolve the clot by fibrinolysis. In DIC, the process of coagulation and fibrinolysis become abnormally, often massively, activated leading to systemic coagulation and fibrinolysis.
Disseminated massive activation of the coagulation cascade leads to production of thrombi (which compose mainly of fibrin and platelets) hence depletion of platelet count. Extensive thrombi formation leads to consumption of clotting factors, platelets and anticoagulation factors (protein S, protein C and antithrombin). This consumptive coagulopathy depletes clotting factors and platelets which can then result in massive bleeding.
Causes of DIC include sepsis, malignancy, liver disease, trauma, transfusion reaction due to ABO mismatch, obstetric complications (e.g., preeclampsia, HELLP syndrome, placental abruption), and VTE [1,2]. DIC starts with exposure to a procoagulant like cancer cells or bacteria products such as lipopolysaccharides or tissue factors [3], trauma where damage to endothelium causes release of procoagulant enzymes/ phospholipids. In cases of DIC where thrombosis is more prevalent, the patients may present with arterial or venous thromboembolism [4].
In Madam RA’s case, she was diagnosed with bilateral lower limb DVT which may have occurred as a combination of reduced mobility as well as DIC triggered by sepsis secondary to bilateral pyelonephritis. DIC is common in bacteria sepsis, with increasing likelihood related to severity of systemic infection [5]. This patient had evidence of bilateral pyelonephritis on CT scan with elevated CRP but blood culture was negative. In terms of pyelonephritis, she seemed to be responding to IV pip-tazocin and gentamicin with a lack of fever.
DIC resulted in massive consumption of coagulation factors and platelets which outpaced their production, as shown by abnormally prolonged clotting times, low fibrinogen levels and thrombocytopenia. Fibrinolysis occur resulting in generation of Fibrinogen Degradation Products (FDP) which inhibit subsequent platelet aggregation and disrupts fibrinogen polymerization as part of clot formation. D Dimer is a product of fibrinolysis and was elevated. FDPs in large quantities effectively act as pathological anticoagulant and anti-platelet agents. DIC often result in spontaneous bleeding diatheses and supportive treatment with transfusions of blood products are usually ineffective. Fortunately, in this case, there were no obvious bleeding diathesis observed such as petechiae, bruising or prolonged oozing after venepunctures. Nevertheless, fresh frozen plasma was given to reduce bleeding risks.
DIC can also cause organ dysfunction due to vascular thrombosis, haemorrhage or hypoperfusion. For RA, she showed evidence of acute kidney injury, high serum lactate levels and retiform purpura of her distal lower extremities on Day 2 of admission. Microangiopathic Haemolytic Anaemia (MAHA) is milder in DIC compared to other thrombotic microangiopathies. This patient had evidence of MAHA as suggested by elevated LDH, haptoglobin and blood film findings of schistocytes and helmet cells.
This case illustrates the challenges of managing VTE with concomitant DIC. Traditionally, first-line treatment for DVT or Pulmonary Embolism (PE) in a hemodynamically stable patient is therapeutic anticoagulation. However, in RA’s case therapeutic anticoagulation was contraindicated due to significant thrombocytopenia and abnormally prolonged clotting times. These were indications of high bleeding risk, and her clotting times and platelet counts remained abnormal despite symptomatic support with blood products.
Thrombolysis may be considered for patients with submassive PE and low bleeding risk [6]. However, thrombolytic therapy achieves only clot lysis at the expense of increased bleeding risk but has no impact on long term outcomes like mortality or recurrence of DVT when compared to anticoagulant [7]. Hence systemic thrombolysis is not routinely recommended for treatment of DVT. Thrombolysis was also unsuitable due to high bleeding risk from underlying DIC [8]. RA’s deterioration occurred suddenly with drop in BP and oxygen saturation. Despite supportive treatment with IV fluid boluses and supplemental oxygen, her vital signs did not improve. There would not have been adequate time to administer the full thrombolytic dose as the massive PE blocked both her pulmonary arteries with evidence of right heart strain [9].
Saddle embolus which causes massive PE is usually a rapidly fatal complication of DVT and comprises 10% of all PE cases [10]. Large enough saddle embolus obstructs both right and left pulmonary arteries, resulting in right heart failure and death.
Mechanical Thrombectomy is an option for DVT or PE in those with high bleeding risks or those who failed thrombolysis and are at low bleeding risk. It is an endovascular procedure involving catheter-directed removal of the thrombus or embolus. Various techniques are available like angioplasty, aspiration, rotational, rheolytic and ultrasound accelerated device [11-13]. A catheter is typically inserted via the right femoral vein or, if contraindicated, the right jugular vein. While primarily used for acute cerebral ischemic stroke, it can also be employed for clot retrieval in acute myocardial infarction and PE [14]. Catheter directed thrombolysis may involve infusion of thrombolytic drug directly into the thrombus. Major bleeding as a complication occurs in 3.6% of patients undergoing thrombectomy. These include intracranial bleeding, gastrointestinal bleeding, retroperitoneal bleeding and haemolytic anaemia requiring transfusion. Minor bleeding complications in 28% of patients were associated with access site bleeding [15].
Mechanical thrombectomy helps restore venous patency and reduces post-thrombotic complications. Ideally, mechanical and/or pharmacological anticoagulation should follow thrombectomy to prevent recurrence. Mechanical Thrombectomy offers significant functional benefits for young patients with acute DVT and is a superior option for managing VTE in high bleeding risk subgroups [16]. Success rate of total or near-total removal of thrombus or embolus can be up to 87% (16) and venous patency rate is 75-100% at 12 months [17].
VTE is associated with a mortality rate of 23% [18]. Untreated acute PE is associated with 30% mortality and mortality is still high at 8% even among treated PE (10). Clinicians should actively identify patients at risk for VTE and initiate mechanical and/or pharmacological prophylaxis to reduce VTE incidence [19]. Diagnosis of VTE remains challenging for most clinicians, as signs are vague. Hence, vigilance for VTE signs and symptoms is crucial, as patients can deteriorate rapidly, especially when PE occurs as a complication. Early detection and initiation of treatment is essential to prevent recurrent thrombosis and mortality [20]. Many medicolegal cases arise from delayed or missed diagnoses of VTE [21]. In cases where traditional treatment options are contraindicated, catheter directed mechanical Thrombectomy can be considered to address the limitations associated with conventional treatment options like anticoagulant therapies. This also highlights the importance of multidisciplinary management in complicated cases of VTE.
Conclusion
This case presented atypically with sepsis complicated by DIC which may have been missed since clinical signs were subtle. Once DIC was diagnosed at the same time as bilateral lower limbs DVT were proven. The challenge of choosing anticoagulation therapy in the face of bleeding diathesis is frequent among cases of DIC. Catheter directed mechanical thrombectomy may have saved her life if saddle embolus was swiftly and successfully removed.
References
- Costello RA, Leslie SW, Nehring SM. Disseminated intravascular coagulation, 2024.
- Levi M, et al. 'Guidelines for the diagnosis and management of disseminated intravascular coagulation,' British Journal of Haematology, 2009; 145(1): pp. 24–33. https://doi.org/10.1111/j.1365-2141.2009.07600.x.
- Hellum M, et al. Microparticle associated tissue factor activity correlates with plasma levels of bacterial lipopolysaharides in meningococcal septic shock. Throm Res, 2014; 133(3): 507-514.
- Gordon SG, et al. Cancer procoagulant: a factor X activator, tumour marker and growth factor from malignant tissue. Blood Coagul Fibrinolysis, 1997; 8(2): 73.
- Smith OP, et al. Use of protein C concentrate, heparin and haemofiltration in meningococcal induced purpura fulminans. Lancet, 1997; 350(9091): 1590.
- Clark D, et al. 'Submassive pulmonary embolism,' Circulation, 2013; 127(24): pp. 2458–2464. https://doi.org/10.1161/circulationaha.112.000859.
- Stevens SM, et al. Antithrombotic therapy for VTE disease: Second update of the CHEST Guideline and Expert Panel Report. Chest, 2021; 160(6): e545.
- Badireddy M, Mudipalli VR. Deep venous thrombosis prophylaxis, 2023.
- Bremer W, Ray CE, Shah KY. 'Role of interventional radiologist in the management of acute pulmonary embolism,' Seminars in Interventional Radiology, 2020; 37(01): pp. 062–073. https://doi.org/10.1055/s-0039-3401841.
- Ata F, et al. 'Optimal management, prevalence, and clinical behavior of saddle pulmonary embolism: A systematic review and meta-analysis,' Thrombosis Research, 2022; 217: pp. 86–95. https://doi.org/10.1016/j.thromres.2022.07.013.
- Murphy KD. Mechanical thrombectomy for DVT. Tech Vasc Interv Radiol, 2004; 7(2): 79.
- Grilli CJ, et al. Rheolytic-accelerated pharmacomechanical directional RAPID thrombolectomy technique:A method for rapid clot removal with reduced need for catheter directed thrombolysis in patients with acute DVT. J Vasc Surg Venous Lymphat Disord, 2014; 2(1): 117.
- Rabuffi P, et al. Pharmacomechanical catheter directed thrombolysis for acute iliofemoral deep vein thrombosis: our case series. Eur Rev Med Pharmacol Sci, 2019; 23(5): 2244.
- Mathews S, De Jesus O. Thrombectomy, 2023.
- Shah KJ, Roy TL. 'Catheter-Directed Interventions for the treatment of lower extremity deep vein thrombosis,' Life, 2022; 12(12): p. 1984. https://doi.org/10.3390/life12121984.
- Wong P, et al. 'Percutaneous mechanical thrombectomy in the treatment of acute iliofemoral deep vein thrombosis: a systematic review,' Hong Kong Medical Journal [Preprint], 2019. https://doi.org/10.12809/hkmj187491.
- Wong PC, et al. Percutaneous mechanical thrombectomy in the treatment of iliofemoral deep vein thrombosis:a systematic review. Hong Kong Med J, 2019; 25(1): 48.
- McCormack T, et al. 'Venous thromboembolism in adults: summary of updated NICE guidance on diagnosis, management, and thrombophilia testing,' BMJ, 2020; p. m1565. https://doi.org/10.1136/bmj.m1565.
- Nicholson M, et al. 'Prevention of venous thromboembolism in 2020 and beyond,' Journal of Clinical Medicine, 2020; 9(8): p. 2467. https://doi.org/10.3390/jcm9082467.
- https://www.uptodate.com/contents/venous-thromboembolism-initiation-of-anticoagulation.
- Wilson E, et al. 'Common reasons for malpractice lawsuits involving pulmonary embolism and deep vein thrombosis,' Journal of Surgical Research, 2020; 245: pp. 212–216. https://doi.org/10.1016/j.jss.2019.07.079.

