The Place of Surgery in the Management of Rectal Cancer
Hajri A, Benzidane K*, Rayadi M, Benjelloun K, El Wassi A, Erguibi D, Boufettal R, Jai S and Chehab F
Department of general surgery, IBN ROCHD University hospital of Casablanca, Casablanca, Morocco
Received Date: 25/07/2024; Published Date: 16/10/2024
*Corresponding author: Benzidane Kamal, Department of general surgery, IBN ROCHD University hospital of Casablanca, Casablanca, Morocco
Abstract
Introduction: Rectal cancer is a common cancer worldwide. Its treatment has made several advances since the introduction of the concept of the total mesorectal excision. However, its prognosis remains poor due to delayed diagnosis.
Objective: The aim of this study was to describe the surgical technique and the department’s experience in this subject, to specify the indications, and to report our surgical treatment results.
Patients and Methods: Our work is a retrospective and descriptive study, involving a series of patients operated for rectal cancer in the digestive cancer surgery and liver transplantation department of CHU IBN ROCHD in CASABLANCA, over a period of 2 years, from January, 2019 to December, 2020.
Results: The average age was 54 years with a sex ratio of 0.95 M/F. The average consultation delay was 6 months. The clinical signs were predominantly rectal bleeding (90.7%). On rectal examination, the tumor was most often located in the lower rectum with 19 cases (44%). Adenocarcinoma was present in 86% of cases. Stage IV was the most frequent with 34.9% of cases. Neoadjuvant concurrent chemoradiotherapy followed by surgery was the strategy adopted in 95.3% of cases. Surgical treatment was conservative in 53.5% and radical in 44.2% of cases. The postoperative course was marked by complications in 18 patients (44.1%). Tumor recurrence was noted in 4 patients (9.3%). Two patients died during follow-up. The follow-up was insufficient to assess 5-year survival.
Conclusion: Rectal cancer surgery has seen many advances, such as minimally invasive surgery with sphincter-preserving techniques for very low-lying tumors. It has also benefited from the contribution of medical treatment that has enhanced its effect. However, the prognosis remains poor, hence the need to develop national screening and prevention strategies.
Keywords: Rectal cancer; Adenocarcinoma; Rectal surgery; Total mesorectal excision
Introduction
According to the latest WHO statistics in 2022, colorectal cancer ranks 3rd among all cancers with a global incidence of 9.6% and a mortality rate of 9.3% [1]. The only potentially curative treatment is surgical resection, particularly total mesorectal excision. This treatment can be radical or conservative and depends on the tumor's location in relation to the anal margin and sphincters, as well as the tumor stage. Neoadjuvant treatment, especially concomitant chemoradiotherapy, has improved the prognosis of rectal cancers by reducing tumor size and recurrence rates [2]. Advances in both surgical techniques and treatment indications have reduced the local recurrence rate at the cost of postoperative morbidity, which is estimated at 20-30%, and have decreased the rate of functional sequelae.
Material and Methods
Our study is a retrospective and descriptive study involving patients who underwent surgery for rectal cancer at the Digestive Oncology Surgery and Liver Transplantation Department of CHU IBN ROCHD in Casablanca over a 2 year period (2019-2020). The objective was to report the various surgical techniques used in the department, as well as the oncological and functional outcomes of the surgery for this cancer.
Results
The number of patients was 43, with an average age of 54 years (ranging from 25 to 83 years) and a sex ratio of 0.95 M/F. More than 50% of the patients had risk factors related to colorectal cancer, with one patient having recto-colic polyposis, and 16% had a family history of neoplasia. The presenting symptoms were primarily rectal bleeding in 39 patients (90.7% of cases) associated with rectal syndrome in 28 patients (65.1% of cases). The general condition was preserved in 90% of patients, with a performance status on 0 or 1 and an average BMI of 22.5 kg/m2.
On clinical examination, the tumor was accessible via digital rectal examination in 35 patients (81% of cases) and located less than 5 cm from the anal margin (AM) in 19 patients (44% of cases), between 5 and 7 cm from the AM in 16 patients (37% of cases), and beyond 7 cm in 8 patients (19% of cases), with sphincter hypotonia in only one patient.
Diagnostic rectoscopy revealed a tumor in the lower rectum in 19 patients, the mid rectum in 18 patients, and the upper rectum in 6 patients.
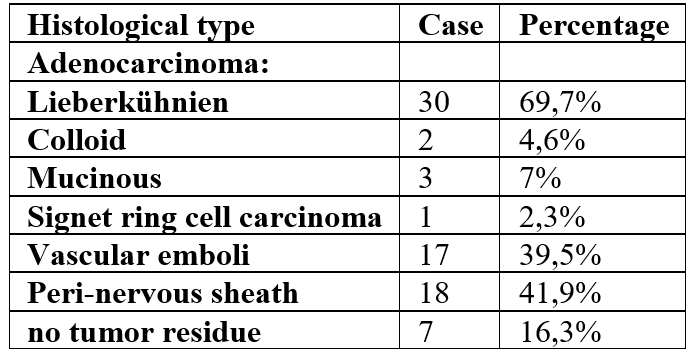
The histopathological study of the biopsies revealed adenocarcinoma in 37 patients, mucinous adenocarcinoma in 3 patients, colloid adenocarcinoma in one patient, while one female patient had signet ring cell adenocarcinoma and there was one case of colloid carcinoma.
Pelvic MRI performed for locoregional staging showed:
Table 1: Pelvic MRI results.
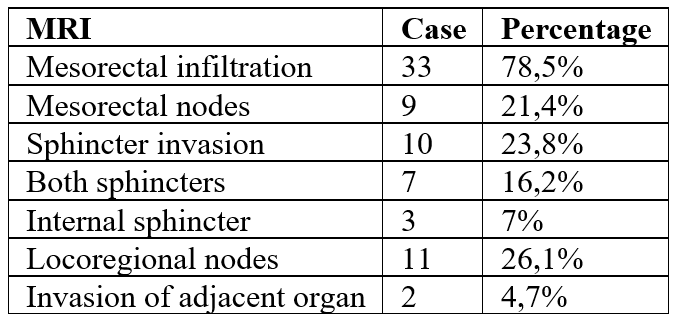
A thoraco-abdomino-pelvic CT scan performed on all patients revealed hepatic metastases in 8 patients (18.6%), pulmonary metastases in 7 patients (16.2%), and peritoneal carcinomatosis in one patient (2.3%).
Colonoscopy, as part of the cancer staging, was incomplete in 18 patients and did not identify any synchronous lesions on others.
All patient records in this series were discussed in multidisciplinary team meetings. Neoadjuvant treatment consisting of radiochemotherapy was indicated in 41 patients, accounting for 95.3% of cases. Radiotherapy (RT) was delivered according to standard protocol (45-50 Gy over 5 weeks), and chemotherapy was administered as Folfox in 12.2% of patients and Capecitabine in 87.8% of patients. The interval between preoperative radiochemotherapy and surgery was 6 to 8 weeks in 88.4% of patients.
Surgical approach was initially laparotomy in 36 patients (83.7%) and laparoscopy in 7 patients, with conversion to laparotomy in 5 patients. Conversion to laparotomy was performed for locally advanced tumors, hypercapnia, and presence of multiple adhesions.
The intraoperative exploration findings are summarized in the table below:
Table 2: Results of intraoperative exploration.

Surgical treatment for curative aim was performed in all patients, with a type of resection determined by the tumor's location. Thus, 23 patients (53.5%) underwent anterior colorectal resection, including one that en bloc included the uterus, 19 patients (44.2%) underwent abdominoperineal amputation, and one patient underwent subtotal colectomy due to associated colorectal polyposis.
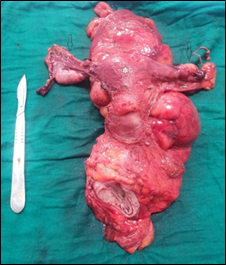
Figure 1: Anterior colorectal resection with colpohysterectomy without adnexal preservation en bloc for a rectal tumor with invasion of the genital tract.
Hepatic metastasectomy was performed on four patients.
Restoration of digestive continuity in patients undergoing conservative treatment consisted of:
- High colorectal anastomosis: 02 cases (8.4%).
- Low colorectal anastomosis: 18 cases (75%).
- Colo-anal anastomosis: 03 cases (12.5%).
- Ileo-anal anastomosis with J-pouch reservoir in one patient (4.1%).
Ileostomy for protection was performed in all patients undergoing conservative treatment (55.8% of cases). Restoration of continuity was performed after endoscopic control of the anastomosis, with an average delay of 16 months.
The results of the histopathological examination of the resection are summarized in the table below (Table 3).
Table 3: The number of lymph nodes (LN) sampled was an average of 8. The resection margins were tumor-positive in only one patient, and the mesorectum was incomplete in 14.2% of cases. Ascites samples revealed the presence of malignant cells in one patient (2.3%).
Adjuvant chemotherapy was administered based on the postoperative tumor stage. It was indicated for 27 patients, accounting for 55.8%, with 2 patients lost to follow-up. Palliative medical treatment was indicated, after surgical treatment, for three patients in this series with a performance status (PS) score of 3.
Regarding morbidity and mortality, one postoperative death was observed in this serie, representing 2.3%. Seventeen patients experienced postoperative complications (detailed in Table IV), resulting in a postoperative morbidity rate of 41.8%.
Table 4: Post operative complications.

During the follow-up of patients, we found:
- Four cases of local recurrence, representing a rate of 9.3%, with an average time of onset estimated at 15 months, ranging from 6 to 24 months.
- Functional complications, particularly sexual, were not reported during patient follow-up.
- Two cases of death within 6 months following the surgical procedure.
- Seven patients were lost to follow-up.
Our follow-up period was insufficient for the majority of patients to calculate 3-year and 5-year survival rates.
Discussion
According to the cancer registry of Casablanca region, the total number of new cases of rectal cancer recorded during the study period (2013-2017) amounted to 846, representing 3.4% of all registered cases [3]. The number of diagnosed cases doubles every decade for patients aged between 40 and 70 for both sexes [4].
Environmental and dietary factors explain the geographical distribution of colorectal cancer (CRC), with high-risk areas including Australia, North America, Western European countries, and Japan [5]. Smoking, chronic alcoholism, a sedentary lifestyle, and obesity are risk factors for the development of CRC and contribute to the formation of advanced adenomas in young individuals. Some studies estimate that 25% of current CRC cases could have been prevented by recognizing family history as a risk factor and initiating high-risk screening [6].
Rectal bleeding and rectal syndrome are the main symptoms of rectal cancer [7]. Digital rectal examination allows access to tumors located in the lower and middle rectum. This clinical evaluation is crucial for establishing a plan for radical or conservative treatment [8].
Rectoscopy with biopsy allows for a definitive diagnosis [9]. A systematic staging is performed to evaluate the extension of the tumor locally, regionally, and distantly, as well as to search for other synchronous primary lesions [10]. This staging is based on a complete clinical examination [11], pelvic MRI, thoraco-abdominal CT with contrast injection, and colonoscopy to search associated lesions [12]. If there is doubt about hepatic lesions on the CT scan, a hepatic MRI with diffusion sequences can better characterize these lesions [13].
A high preoperative level of tumor markers, particularly CEA and CA 19.9, is associated with a poor prognosis [14]. All of these clinical and paraclinical elements allow the classification of the tumor according to the current standard, which is the 8th edition of the Union for International Cancer Control (UICC) classification [15].
This step is followed by an assessment of the patient's operability and surgical risks. It is recommended to refer to the American Society of Anesthesiology (ASA) classification [16].
The therapeutic management of rectal cancer is multidisciplinary, with surgery being the mainstay. A collaboration between the different healthcare professionals involved leads to an improvement in the quality of the treatement [17]. The decision on the therapeutic approach for each patient should be made in a multidisciplinary team meeting.
The goal of neoadjuvant treatment is to reduce the tumor size, leading to a downstaging of rectal cancer, thereby increasing the resectability rate and decreasing the recurrence rate [18]. Radiotherapy has demonstrated a significant effect on reducing local recurrences by nearly 50%. A Swedish study highlighted an increase in overall five-year survival from 48% to 58% [19]. Chemotherapy administered concomitantly with radiotherapy significantly reduces the risk of local recurrence. Various agents and therapeutic regimens are used, either as neoadjuvant treatment in combination with radiotherapy and/or as adjuvant treatment [20].
However, surgery remains the standard treatment for rectal cancer. The type of surgical resection depends on several key factors, including the tumor's location relative to the anal margin and the locoregional extent on MRI [21]. Lower rectal cancers exhibit distal parietal extension beyond the tumor's inferior pole, corresponding to a submucosal tumor extension or tumor emboli of 1 cm, and rarely exceed this distance. Therefore, a distal resection margin of 1 to 2 cm is sufficient [22]. Total mesorectal excision, performed under direct vision without any manual dissection, has improved oncological outcomes by significantly reducing the local recurrence rate.
Several types of anastomoses can be performed depending on various factors, particularly the type of resection. These anastomoses should preferably be protected by an ileostomy, especially in the case of sub-Douglas (subperitoneal) anastomoses, with continuity restoration 4-6 weeks later.
The presence of lymph node metastases is a significant prognostic factor for recurrence and survival [23]. It is necessary to histologically examine at least 12 lymph nodes to properly stage the tumor [24]. Surgical advances have reduced postoperative mortality from 10% to 2% and local recurrence rates from 30-40% to less than 15% [25]. This rate is also affected by the type of surgical intervention. Mortality is lower after anterior resection compared to abdominoperineal amputation (APA) and is decreased after curative resection versus palliative surgery [26].
Intraoperative complications are more often related to hemorrhage from an injury to the presacral venous plexus during posterior rectal dissection, highlighting the importance of extrafascial dissection while respecting the rectal fascia [27]. Ureteral injuries, with an incidence ranging from 0.1% to 4.5%, commonly occur at the level of the pelvic ureter and are more frequent during abdominoperineal amputations.
Immediate complications of rectal resection surgery primarily include anastomotic leaks in the form of fistulas managed by drainage, pelvic abscesses (localized peritonitis), or generalized peritonitis. Functional urinary and sexual complications, related to hypogastric nerve plexus injury, may also occur [28]. Post-anastomotic stenoses are uncommon, particularly with manual anastomosis. They can result from a previous fistula or tumor recurrence. A biopsy at the anastomosis site is essential to differentiate between the two.
Risk factors for recurrence include a positive circumferential resection margin, extramural vascular invasion, lower rectal tumors, and the absence of neoadjuvant treatment. The challenge of rectal cancer has long been the high rate of local recurrences, estimated at 32% [29]. However, since the advent of neoadjuvant therapies and total mesorectal excision, local recurrences have significantly decreased to currently around 4-8%. Thus, an increase in CEA levels during follow-up may indicate a recurrence, and pelvic MRI with diffusion sequences offers better performance for detecting these tumor recurrences.
Conclusion
Improving surgical practices while adhering to oncologic surgery principles for rectal cancer aims to reduce functional sequelae and enhance quality of life. Preserving sphincter function is currently the gold standard, and maintaining sexual and urinary functions is now a priority. However, functional sequelae following curative treatment of lower and middle rectal cancers remain significant.
References
- Globocan, 2022.
- Cotte E, Artru P, Bachet JB, Benhaim L, Bibeau F, Christou N, et al. Thésaurus National de Cancérologie Digestive, 2023.
- Benider A, Harif M, Karkouri M, Quessar A, Sahraoui S, Sqalli S. Registre des cancers de la région du grand Casablanca pour la période, 2008-2012; Ed 2016: 62-72.
- Teyeb E. Profil Epidémiologique Des Cancer Digestif Au Service Chirurgie Visceral, 2021.
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol, 2001; 2(9): 533‑543.
- Stanich PP, Pelstring KR, Hampel H, Pearlman R. A high percentage of early-age onset colorectal cancer is potentially preventable. Gastroenterology, 2021; 160: 1850–1852.
- Holtedahl K, Borgquist L, Donker GA, et al. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: a prospective cohort study of diagnostic accuracy in primary care. BMC Fam Pract, 2021; 22: 148. 10.1186/s12875-021-01452-6.
- Gérard JP AT, Bibeau F, Conroy T, Legoux JL, Portier G. Cancer du rectum. Thésaurus National de Cancérologie Digestive Actualisé, 2021.
- El Hairech D, Achour A. Cancer du rectum: aspect clinique et thérapeutique dans le servicede Chirurgie générale à l’hôpital militaire de Marrakech, Thèse N, 2013.
- Recommandations De La Federation Francophone De Cancerologie Digestive Que faire devant uncancer digestif en 2003? Gastroenterol Clin Biol, 2002; 26: 1140-1164.
- Mariette C, Alves A, Benoist S, Bretagnol F, Mabrut JY, Slim K. Soins périopératoires en chirurgie digestive. Journal de Chirurgie, 2005; 142(1): 14‑28.
- Horvat N, Petkovska I, Gollub MJ. MR Imaging of Rectal Cancer. Radiologic Clinics of North America, 2018; 56(5): 751‑774.
- Yu J, Xu Q, Huang DY, Song JC, Li Y, Xu LL, et al Prognostic aspects of dynamic contrast-enhanced magnetic resonance imaging in synchronous distant metastatic rectal cancer. Eur Radiol, 2017; 27(5): 1840‑1847.
- Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EMK, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. The Lancet Oncology, 2021; 22(1): 29‑42.
- Cotte E, Artru P, Christou N, Conroy T, Doyen J, Fabre J, et al. Cancer du rectum. Thésaurus National de Cancérologie Digestive, 2019.
- Bennani O. Cancer du rectum: Expérience du service de chirurgie viscérale de l’hôpital militaire Moulay Ismail de Meknès , Fès 2014 Thèse N° 100/14 .
- Orsini G, Wiggers T, De Ruiter MC, Quirke P, et al. The modern anatomical surgical approach to rectal cancer J C supplements, 2013; 1(1).
- Vendrely V, Denost Q, Charleux T, Brouquet A, Huguet F, Rullier E. La radiothérapie des cancersdu rectum : stratégie thérapeutique et perspective. Cancer/Radiothérapie, 2018; 22(6): 558‑563.
- Abdalla S, Benoist S, Lefèvre JH, Penna C, Brouquet A. Nouvelles stratégies de prise en charge du cancer du rectum non métastatique. Journal de Chirurgie Viscérale, 2021; 158(6): 546‑556.
- Michel P, Di Fiore F. Chimiothérapie du cancer du rectum. Cancer/Radiothérapie, 2011; 15(6‑7): 436‑439.
- Ghouti L, Portier G, Kirzin S, Guimbaud R, Lazorthes F. Traitement chirurgical des récidiveslocorégionales du cancer du rectum. Gastroentérologie Clin Biol, 2007; 31(1): 55‑67.
- Dumont F, Mariani A, Elias D, Goéré D. Surgical strategy for low rectal cancers Département. Journal of Visceral Surgery, 2014.
- Sevá-Pereira G, Cypreste RN, Oliveira Filho JJ, Moraes SP de, Tarabay PB. Recurrence pattern of rectal cancer after surgical treatment. Analysis of 122 patients in a tertiary care center. Journal of Coloproctology, 2018; 38(01): 018‑23.
- Glynne-Jones R, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 2017; 28 (Supplement 4); iv22–iv40.
- Fleming FJ, Påhlman L, Monson JR. Neoadjuvant therapy in rectal cancer. Dis Colon Rectum, 2011; 54: 901–912.
- Ibzer S. Traitement chirurgical du cancer du bas et moyen rectum Thèse de doctorat en médecine, FMPM, Année 2018 Thèse N°145.
- Machado M, Nygren J, Goldmann S, Ljungqvist O. Functional and physiologic assessment ofthe colonic reservoir or side-to-end anastomosis after low anterior resection for rectal cancer: a two-year follow-up. Dis Colon Rectum, 2005; 48: 29–36.
- Abdelli A, Tillou X, Alves A, Menahem B. Séquelles génito-urinaires après résection rectale carcinologique. Que dire aux patients en 2017 ? Journal de Chirurgie Viscérale, 2017; 154(2): 99‑110.
- Pimentel JM, Duarte A, Gregorio C, Souto P, Patricio J. Transverse coloplasty pouch andcolonic J-pouch for rectal cancer: a comparative study. Colorectal Dis, 2003; 5: 465–470.

