Metastatic Forms of Differentiated Thyroid Cancer
Amal Hajri, Mahassine Rayadi*, Kamal Benzidane, Driss Errguibi, Rachid Boufettal, Saad El Jay and Farid Chehab
Department of General Surgery, IBN ROCHD University Hospital Center, Casablanca, Morocco
Received Date: 24/07/2024; Published Date: 16/10/2024
*Corresponding author: Mahassine Rayadi, Department of General Surgery, IBN ROCHD University Hospital Center, Casablanca, Morocco
Abstract
Differentiated thyroid carcinoma (DTC), including papillary and follicular carcinoma, is the most common malignant endocrine neoplasm in adults, accounting for over 90% of all thyroid cancers. Characterized by often localized or regional extension, it generally has a favorable prognosis. Distant metastases primarily involve the lungs and bones. Other localizations are rare (10%). Their occurrence considerably worsens the prognosis. Objective to study the clinical, paraclinical, histological, therapeutic and evolutionary aspects of metastatic CDT. This descriptive and retrospective study of 78 patients from 762 files of patients followed in our entity for differentiated thyroid cancer during 34 years from January 1986 to 2020 having presented metastases. Mean age 46.4 (10-80) years with a female predominance in 90% of cases. Total thyroidectomy without lymph node dissection was performed in 51% of cases. The mean duration of recurrence as distant metastasis was 43.9% months after surgery. Papillary carcinoma was dominant in 85% of cases. Capsular effraction was found in 34% of cases, vascular emboli in 16%, with multifocality in 23% of cases. Ira therapy was indicated in 97% of patients. The metastases were located in the brain (2 cases), lung (15 cases), bone (18 cases), lymph nodes (60 cases) and skin, sternum, larynx and retro-orbital (4 cases). The prognostic factors for recurrence were age greater than 55 years, advanced TNM stage, capsular invasion and multifocality. The recurrence rate of differentiated thyroid cancers is low in our series. The risk of recurrence is correlated with several prognostic factors such as advanced age, multifocality, capsular invasion and vascular emboli.
Keywords: Differentiated thyroid cancer; Metastasis; Iodine; Treatment
Introduction
Differentiated Thyroid Carcinoma (DTC), including papillary and follicular carcinoma, is the most common malignant endocrine neoplasm in adults and accounts for more than 90% of all thyroid cancers [1].
Differentiated thyroid cancer is the most common of endocrine cancers but nevertheless remains rare, representing only 1% of all cancers [2,5] . Its incidence has been increasing in recent decades worldwide [3,6].
Metastases can occur in 10% of cases, worsening the prognosis, the preferred locations of which are the bone and the lung [4,8]. They can be revealing or appear during follow-up. The management of metastases is multidisciplinary. It depends on the location of metastases and associated prognostic factors [5].
The objective of this work was to determine the different epidemiological, clinical, histological and progressive aspects of metastatic differentiated thyroid carcinomas, as well as the treatment and monitoring modalities.
Meterial and Methods
Patients: A cohort study was carried out with a retrospective review of the files of seventy-eight patients followed for differentiated thyroid cancer in the endocrinology-diabetology and metabolic disease department of the Ibn Rochd university hospital center in Casablanca. This study was carried out over a period of 34 years from January 1986 to March 2020. The surgical treatment was carried out in the general surgery departments (wing III) of the Ibn Rochd University Hospital in Casablanca and the Oto-Rhino-Laryngology departments of the hospital. August 20 from Casablanca.
We included in this study all patients initially treated for a thyroid tumor, and for whom the histological and immunohistochemical study of biopsies and surgical specimens had confirmed that it was a metastasis of a differentiated thyroid carcinoma. , either during the initial diagnosis, or during its evolution.
Evaluation Methods: During this work, we collected epidemiological data (age, sex, history), clinical data (circumstances of discovery), histological data (type, unfavorable histological elements such as location) through a data sheet. multifocal thyroid, breakage of the capsule of the thyroid nodule and the presence of peritumoral vascular emboli, the size of the tumor, TNM stage), biological, paraclinical and progressive of the patients.
Statistical tools: SPSS version 20 software was used for the analysis of parametric data. Univariate analyzes were performed by calculating measures of central tendency and dispersion based on the distribution characteristics of each of the quantitative variables, while categorical variables were presented in absolute and relative frequencies. Bivariate analysis of the association between clinical and histological variables and regression results was estimated using the chi-square test or Fisher's exact test for categorical variables, and the Mann-Whitney test or the t test for continuous variables, with the calculation of relative risk (RR) values. The p value < 0.05 was statistically significant.
Results
The average age of our patients was 46.4 years with a range of 10 to 80 years, predominantly female with an F/M sex ratio of 8.75 (Figure 1)?
A history of oncological disease was noted in 7 patients:
- Two cases of breast cancer,
- A case of cavum cancer
- Four cases of familial papillary carcinoma.
The most frequent reason for consultation was thyroid nodules in 75.6% of cases, most often in the form of GMHN (48.7%). Furthermore, metastases were revealing in 19 cases or 24.3% including 5.12% of cases at the lymph node level and 19.1% of distant locations. Total thyroidectomy was indicated in all patients except one patient, due to tumor adhesion and pulmonary morbidity. Lymph node dissection was performed in 50% of cases.
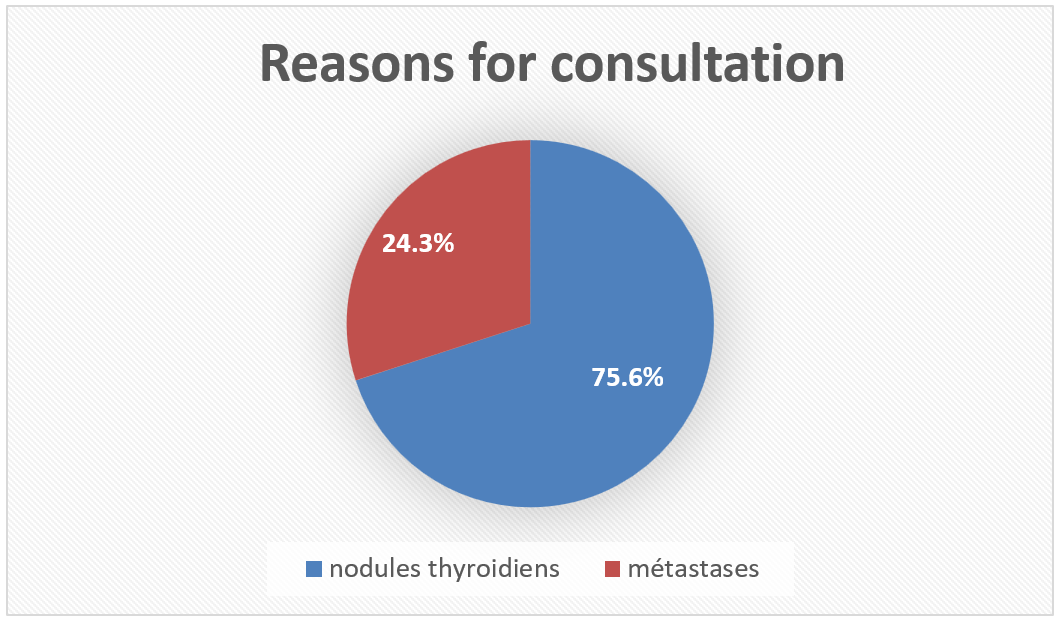
Figure 1: Distribution of patients by age group.
Histological examination revealed 11% gallbladder carcinoma and 89% papillary carcinoma, including 9 cases of papillary thyroid microcarcinomas (12%) and lymphocytic thyroiditis was only associated in 2 patients. Lymph node invasion was the majority in both histological types with 79% for gallbladder carcinoma and 89% for papillary carcinoma. According to the TNM classification, 24% of cases were classified pt1, 25% of pt2 cases, 15% of pt3 cases, 11% of pt4 cases, and the remaining 25% were unspecified.
For unfavorable histological signs, we found:
- Multifocal thyroid locations in 23% of cases
- A breakage of the capsule of the thyroid nodule in 34% of cases
- Vascular neoplastic emboli were found in 16% of cases.
Cervical ultrasound was performed in all patients. It revealed recurrent thyroid nodules in 10 patients, a parathyroid nodule in 1 patient and suspicious cervical lymphadenopathy in 60 patients.
The thyroglobulin level after total thyroidectomy was greater than 100 ng/ml in 65 patients, with a maximum of 16,000 ng/ml. Anti-thyroglobulin antibodies were present in 9 patients.
Metastases predominated at the lymph node level in 60 cases (77%), while 18 cases had bone metastases (23%), 15 had pulmonary metastases and 6 had rarer metastases, notably brain (2 cases), skin, parathyroid, laryngeal and 1 retro-orbital case. Multifocal involvement was found in 5 patients.
Surgical treatment of distant metastases was carried out in 15 patients (19.2%). It was about :
- Tumor excision for retro-orbital, cutaneous, parathyroid metastasis
- Total excision of the bone tumor in 12 patients (15.3%)
The anatomopathological study with immuno-histochemical complement, surgical specimens and biopsies was carried out in all our patients who confirmed the thyroid origin of the metastases.
Iodine 131 iratherapy was indicated in all our patients, except one patient in whom the thyroid carcinoma was not operable.
Hormone therapy at a restraining dose was systematic for all of our patients with L-Thyroxine either post-operatively or after Iratherapie.
Monitoring of all patients during the first year was frequent, then biannual, then annual and continued for life.
Complete remission was noted in 60 cases or 64%, while 12 patients (15%) are still in a stable condition. However, 6 cases of death were noted, i.e. 7.7%, mainly due to the progression of the metastatic disease. The follow-up in our series was 34 years.
Discussion
Differentiated thyroid cancers are the most common malignant epithelial tumors of the thyroid body that arise from follicular cells exhibiting certain morphological and functional characteristics similar to that of normal thyroid tissue. They represent papillary cancers, the most common (80%) and follicular cancers [8].
The thyroid nodule is the most common manifestation of thyroid cancer. It is often asymptomatic and clinically detectable in 4 to 7% of the general population; their frequency increases with age, particularly in women [9,10]. Thyroid nodules are rarely isolated and are most often part of a GMHN. The risk of cancer is similar whether it is a solitary nodule or a GMHN. In our study, the majority of patients presented for nodular goiter, i.e. 48.7%.
Cervical lymphadenopathy is often a mode of metastatic revelation of thyroid cancer, even more rarely, it involves pulmonary or bone metastasis and confirmation of the diagnosis is essentially based on the demonstration of thyroglobulin by immunohistochemistry on the biopsy or excision of metastatic lesions [2,6].
Differentiated thyroid cancers are usually limited to the thyroid gland. Rates of distant metastases vary between 4 and 15%[1,18]. In our study these metastases were synchronous in 10% of cases and metachronous in 18% of cases. These results are close to other studies….
The location and frequency of distant metastases vary depending on the histological type. The mode of dissemination is different in the two types of cancer. Papillary cancer is known to metastasize via the lymph nodes, while follicular cancer metastasizes hematogenously, which explains its distant spread compared to papillary thyroid cancer [9,10].
In addition to the lymph nodes, the most common sites of metastases are the lungs and bone, however involvement of other organs is possible of which it represents < 5% [6].
In our patients, it was rather lymph node metastases which were the most frequent with a percentage of 77%, while bone and lung metastases represented only 23% and 20% respectively, the other sites were 7.7%.
Advanced age, high TNM stage, capsular breakage and multifocality are poor prognostic factors identified in our study. These results are consistent with the work of other studies [1,4], which indicate that age greater than 55 years, aggressive histopathological characteristics and local tumor extension or distant metastases are indicators of poor prognosis.
Total thyroidectomy from the outset is the standard treatment of choice for metastatic CDT. In our series, all patients had undergone total thyroidectomy except one due to tumor adhesion.
Total thyroidectomy with lymph node dissection is recommended for CDT with locoregional extension, according to the guidelines of the American Thyroid Association (ATA)[1,4,18] but the indications and extent of cervical lymph node dissection in thyroid cancer are still controversial, with the possibility of very different attitudes, particularly with regard to prophylactic cures. It is recommended to carry out a dissection if suspicious cervical lymphadenopathy is identified pre- or intra-operatively in order to reduce the risk of recurrence in low-risk patients and to improve survival in high-risk patients. [12].
Iratherapy remains the essential element in the treatment of distant metastases of the CDT after total thyroidectomy [13,14,15]. Its objective is:
- Treat possible macro or microscopic post-operative tumor foci.
- Destroy normal thyroid tissue to improve the sensitivity of subsequent monitoring (thyroglobulin dosage);
- Carrying out the extension assessment using whole body scintigraphy carried out 3 to 7 days after the iodine dose.
This treatment is administered in the form of an iodine 131 capsule at a dose of 3.7 GBq/course after stimulation of TSH. The latter is achieved by weaning the hormonal treatment for 3-4 weeks or after two injections of exogenous human recombinant TSH (thyrotropin alfa, Thyrogen) 24 hours apart. The effectiveness of this treatment depends on the ability of the tumor tissue to fix radioactive iodine and the volume of the tumor tissue. The iodine 131 courses are repeated every 6 to 12 months, then more spaced apart, as long as significant fixation persists and there is a morphological and/or biological and/or scintigraphic response without exceeding a dose of 22 GBq. Pulmonary micrometastases represent the most favorable situation for observing a cure thanks to iodine 131. Unlike pulmonary macrometastases and bone metastatic locations where we note an analgesic effect and stabilization of the lesions.
When the metastatic lesions are immediately non-fixing or iodo-fixing, progressing despite treatment with IRA therapy and persistent after treatment with an iodine 131 dose of 22GBq, they are considered refractory [6,9]. Although radioactive iodine ablation (RAI) therapy is the first-line treatment for metastatic disease, one third of patients do not absorb 131I or become refractory to RAI [3,6,16]. In the latter group, alternative treatments such as external beam radiotherapy, multikinase inhibitors and targeted therapies for tumors with specific molecular alterations have mainly led to an increase in the time to progression [7–10]. Given that almost a third of patients with metastatic disease may remain stable for up to 10 years or more [3, 11], active surveillance is a rational approach to patient care while limiting surgical or pharmacological interventions to those with progressive disease or mass effect symptoms.
Patient monitoring is done through clinical examination, serum thyroglobulin measurement, cervical ultrasound and iodine 131 scintigraphy. The 10-year survival rate in the presence of metastases varies from 25 to 40% [7]. It is more favorable in papillary, well-differentiated forms whose chest x-ray image is normal or miliary type, occurring before the age of 45 and respond to iodine 131.
In our series, 97% of patients received iratherapy, reflecting this standard approach.
Conclusion
Differentiated thyroid cancer has a good prognosis. However, 10% of patients with differentiated carcinomas will present with locally advanced disease. Metastases being the main cause of death.
The principles of treatment depend on the stage of the cancer, the location and extent of the metastasis and consist of a total thyroidectomy with lymph node dissection, treatment with radioactive iodine, slowing treatment and surgical treatment of the metastasis if indicated.
The risk of recurrence is frequent and can occur several years after treatment, hence the importance of long-term monitoring.
Conflicts of interest: The authors declare no conflict of interest.
Author contributions: All authors read and approved the final version of the manuscript.
References
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Guidelines for the Management of Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Cancer differentiated from the thyroid. Thyroid, 2016; 26: 1-133.
- Chen D, Huang L, Chen S, Huang Y, Hu D, Zeng W, et al. Innovative analysis of distant metastases in differentiated thyroid cancer. Oncol. Lett, 2020. doi:10.3892/ol.2020.11304.
- Hirsch D, Levy S, Tsvetov G, Gorshtein A, Slutzky-Shraga I, Akirov A, et al. Long-term outcomes and prognostic factors in patients with differentiated thyroid cancer and distant metastases. Endocr. Pr. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol, 2017; 23: 1193.
- Angélica María González-Clavijo, Andrés A Cuellar, Jenny Triana-Urrego, Jorge A Barrero, Luis Felipe Fierro-Maya. Metastatic differentiated thyroid cancer: worst prognosis in patients with metachronous metastases, Endocrine, 2023; 81(1): 90–97.
- Mihailovic J, Stefanovic L, Malesevic M. Differentiated Thyroid Carcinoma with Distant Metastases: Probability of Survival and Its Predicting Factors. Cancer Biother Radiopharm. Avr, 2007; 22(2): 250‑255.
- Larwanou MM, Houda S, El Ouahabi H, Alaoui NI. Cancer différencié de la thyroïde métastatique à propos de 70 cas : impact des nouvelles recommandations de 2017. Médecine Nucl. Janv, 2020; 44(1): 12‑17.
- Chiofalo MG, Setola SV, Gennaro FD, Fulciniti F, Catapano G, Losito NS, et al. Follicular thyroid carcinoma with skull metastases. Endocr J, 2015; 62(4): 363‑369.
- Schlumberger M. Cancer papillaire de la thyroïde : vers une désescalade thérapeutique. Bull Académie Natl Médecine. Avr, 2017; 201(4‑6): 699‑706.
- Schlumberger M. Cancer papillaire et folliculaire de la thyroïde. Ann Endocrinol. Juin, 2007; 68(2‑3): 120‑128.
- Goffredo P, Sosa JA, Roman SA. Differentiated Thyroid Cancer Presenting with Distant Metastases: A Population Analysis Over Two Decades. World J Surg. Juill, 2013; 37(7): 1599‑1605.
- Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, et al. Bone Metastases from Thyroid Carcinoma: Clinical Characteristics and Prognostic Variables in One Hundred Forty-Six Patients. Thyroid. Mars, 2000; 10(3): 261‑268.
- Schmidbauer B, Menhart K, Hellwig D, Grosse J. Differentiated Thyroid Cancer-Treatment: State of the Art. Int J Mol Sci, 2017; 18(6): 1292.
- Zerdoud S, Leboulleux S, Clerc J, Leenhardt L, Bournaud C, Al Ghuzlan A, et al. Traitement par iode 131 des cancers thyroïdiens différenciés : recommandations 2017 des sociétés françaises SFMN/SFE/SFP/SFBC/AFCE/SFORL. Médecine Nucl, 2017; 41: S1‑22.
- Qiu Z-L, Shen C-T, Luo Q-Y. Clinical Management and Outcomes in Patients with Hyperfunctioning Distant Metastases from Differentiated Thyroid Cancer After Total Thyroidectomy and Radioactive Iodine Therapy. Thyroid. Févr, 2015; 25(2): 229‑237.
- Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf), 2005; 63(1): 87‑93.
- Leite AKN, Kulcsar MAV, de Godoi Cavalheiro B, de Mello ES, Alves VAF, Cernea CR, et al. Death related to pulmonary metastasis in patients with differentiated thyroid cancer. Endocr. Pr. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol, 2017; 23: 72–78. doi: 10.4158/EP161431.
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet Lond. Engl, 2014; 384: 319–328. doi: 10.1016/S0140-6736(14)60421-9.
- Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol, 2021 ; 9: 491–501.
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet Lond. Engl, 2014; 384: 319–328. doi: 10.1016/S0140-6736(14)60421-9.
- Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol, 2021; 9: 491–501.

