Rare Entity: Metachronous lung adenocarcinoma in a single patient harbouring distinct EGFR mutation status
You Zhi Ho1, Chan Siang Kan1,*, Tze Sen Chang1, Adam Malik2, Yuan Hsun Jong1 and Sing Yang Soon1
1Department of Cardiothoracic Surgery, Sarawak Heart Center, Malaysia
2Department of Pathology, Sarawak General Hospital, Malaysia
Received Date: 13/07/2024; Published Date: 11/10/2024
*Corresponding author: Chan Siang Kan, Department of Cardiothoracic Surgery, Sarawak Heart Center, Malaysia
Abstract
Background: Recurrence of lung adenocarcinoma is common during the surveillance period. However, it is uncommon to detect two lung tumor lesions that exhibit different identities according to the epidermal growth factor receptor (EGFR) mutation. We report a case of metachronous lung adenocarcinoma in a 66-year-old man who was first diagnosed with metachronous right lung adenocarcinoma harboring distinct EGFR status expressions.
Case Presentation: We present a case of an elderly gentleman with a known case of the RML adenocarcinoma diagnosed in March 2018 who underwent right video-assisted thoracoscopic surgery (VATS), middle lobectomy, and lymph node resection. Histopathological examination of the specimen revealed adenocarcinoma without an EGFR mutation. He was subsequently placed under oncological treatment. During surveillance, a new spiculated nodule with a size of 1.9 cm largest diameter over the anterior segment of the RUL was detected on computed tomography (CT). An endobronchial ultrasound (EBUS) transbronchial biopsy (TBLB) later showed adenocarcinoma with positive EGFR mutation [exon 21.p(L858R]. The patient underwent surgical resection with curative intent after multidisciplinary discussion.
Discussion: Tumors that exhibit distinct identities related to immunochemistry and molecular testing is a rare identity. Hence, it is difficult to predict the recurrence of a tumor or tumor de novo unless the specimen is subjected to further histopathological examination, immunochemistry, or immunotyping. It is important to differentiate the second lesion whether it is primary in origin or metastasis by targeted biopsy over the lesion (EBUS / Imaging guided biopsy) is crucial, and initiation of preoperative adjuvant chemotherapy/immunotherapy is required by identifying immunohistochemistry. Neoadjuvant immunotherapy targeting specific mutations ( EGFR, ALK, K-RAS) has also been proven to improve the survival benefit of patients receiving tyrosine kinase inhibitors. Surgical resection remains the gold standard for respectable metachronous lung cancer with no evidence of distant metastasis to improve survival outcomes.
Conclusion: Accurate differentiation of primary tumors from metastases through detailed testing is essential for effective treatment. Targeted biopsies and neoadjuvant therapy can improve survival outcomes, with surgical resection being the preferred approach for resectable cases without distant metastasis.
Keywords: Lung Cancer; Metachronous; EGFR mutation
Introduction
According to the 2014 World Health Organization report, lung cancer accounted for 19.1 deaths per 100,000 population in Malaysia or 4,088 deaths per year (3.22% of all deaths), which is the second most common cause of death due to cancer in the country after breast cancer and the eighth most common cause of death from all causes [1]. The classification of lung cancer types is in accordance with the latest revised World Health Organization (WHO) report in 2015, which include adenocarcinoma, adenosquamous cell carcinoma, squamous cell carcinoma, large cell carcinoma, sarcomatoid carcinoma, neuroendocrine carcinoma, and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia I [2].
It is unusual to encounter a patient with dual pathology in which both primary lung cancers occur at different time intervals and exhibit different expressions of the mutation of molecular markers (Epidermal Growth Factor Receptor, EGFR), which may be directed to different modalities of immunotherapy or chemotherapy. The development of high-quality Computed Tomography (CT) imaging over the past decade and close regular CT surveillance programs have played a crucial role in identifying recurrence and the occurrence of new pathologies. Due to improved lung cancer screening and more advanced, detailed imaging, current imaging enables an earlier detection of tumors.
Multiple Primary Lung Cancer (MPLC)
Multiple Primary Lung Cancer (MPLC) is defined as two or more primary lung cancers occurring in the same patient. Generally, MPLC can be classified as synchronous multiple primary lung cancer (sMPLC) and metachronous multiple primary lung cancer (mMPLC) [3]. Metachronous lung cancer was defined as any Non-Small Cell Lung Cancer (NSCLC) occurring after a prior curative lung resection regardless of disease-free interval (DiFI), tumor location, or histologic type [5]. The risk of developing a new primary lung cancer after undergoing definitive surgical therapy for an NSCLC is estimated to be 1%–2% per patient per year [7,8]. This risk is cumulative over time. For example, in two large series of patients with resected early-stage lung cancer followed for 4.2 and 5 years, respectively, 8.6% and 11.7% of patients experienced MLC [9,10]. The incidence of metachronous lung cancer has increased in recent years as a result of longer survival after resection of primary lung cancers, with a reported incidence of 2% to 6% per patient per year of follow-up [4-6]. The primary concern in this situation is how one differentiates or distinguishes MPLC from Intrapulmonary Metastasis (IM) in patients with prior NSCLC. This remains a challenging task. Among patients who present with second lesions, differentiating intrapulmonary metastases and recurrent lung cancer from MPLC can be difficult [5].
Diagnosis
In patients who present with a second lung nodule or mass, distinguishing initial cancer recurrence from second primary cancer remains a challenging task, especially when the patients share the same tumor histology. Previous studies have proven histological type and disease-free interval as criteria for diagnosing metachronous lung cancer. The Martini–Melamed criteria is one of the traditional criteria that most clinicians use. A tumor is considered metachronous second primary cancer if the histologic type was discordant or if the Disease-Free Interval (DFI) was at least 2 years for tumors with a cell type similar to that of the index primary, or if the tumor is associated with carcinoma in situ or arises in a different lobe without common lymphatics [9].
Some authors have modified this to at least a four-year interval between histologically identical cancers, and an interval of two to four years represents a gray area where it is difficult to decide whether a new lesion is a second primary [11-13]. Benjamin E Lee et al. proposed that Martini and Melamed Criteria have little practical or therapeutic utility in evaluating these patients preoperatively [5]. No consensus has been achieved yet among the Union for International Cancer Control (UICC), American Joint Committee on Cancer (AJCC), and International Association for the Study of Lung Cancer (IASLC). The International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Project has recently proposed clinicopathological criteria to improve this distinction and improve classification consistency. However, there is a substantial rate of misclassification using these criteria and a need for a multidisciplinary approach to consider all available information in the final staging. [14] Therefore, decisions based on clinical and pathological data lack the necessary resolution to classify certain multiple lung cancers into either category, and they may be subjective and affected by interobserver variability among chest physicians and pathologists [15].
Molecular Testing
Comprehensive histological assessment of surgical specimens has also been reported to be a reliable method for differentiating second primary cancer from recurrent tumor and has shown good reproducibility among lung pathologists [16,17]. However, differentiation between lesions can be challenging when multifocal tumors are histologically identical [18]. In general, tumors with largely concordant mutations have been considered as clonal in origin (metastases), and those with discordant mutations are considered primary tumors. Many molecular techniques have been reported to be effective for this purpose. The molecular analysis of somatic changes within tumor deoxyribonucleic Acid (DNA) has emerged as a supplement for increasing the reliability of defining lineage of MPLC [19].
Patients with lung adenocarcinomas are now routinely tested for a panel of oncogenic driver mutations (such as Kirsten Rat Sarcoma [KRAS], Epidermal Growth Factor Receptor [EGFR], and Anaplastic Lymphoma Kinase [ALK]) in the clinical setting. These mutations are identified not only for the selection of precision therapy but are of significant interest in the study of metachronous or synchronous lung adenocarcinomas, as they are involved in early-stage tumorigenesis before clonal expansion [20]. The same mutations may occur in morphologically different tumors and driver mutations, especially for EGFR germline mutation, which can also complicate the interpretation of the clonal relationship of multiple lung adenocarcinoma [19,23].
Quéré et al. reported a discordance between the mutational status of primary lung cancers and their metastases. However, given the stability of EGFR-activating mutations, discordance mainly concerned KRAS, an oncogene frequently mutated in lung cancer, particularly in smokers. A major issue raised by targeted therapies is potential discordance between the mutational status of the primary tumor and its metastases or between two regions of the same tumor. However, metastatic sites are rarely biopsied in real-life settings [24].
Discordant responses to chemotherapy or tyrosine kinase inhibitors have also been reported between primary tumors and their metastases, suggesting the existence of biological differences. Next-generation sequencing with comprehensive molecular analysis may help differentiate MPLC from IM [21,22]. Previous studies have reported that as many as 32% of all histologically confirmed synchronous lung tumors have been misclassified as IPM compared with molecular analysis.
Case Report
We report a case of metachronous lung adenocarcinoma in a 66-year-old man who first presented with chronic cough for 3 months and worsening exertional dyspnoea in October 2017. He denied any constitutional symptoms. Chest radiography showed patchy, ill-defined opacity at the right middle zone (Figure 1). Subsequently, contrast-enhanced computed tomography (CT) of the thorax revealed a spiculated heterogenous mass at the medial segment of the right middle lobe measuring 2.9 x 4.1 x 2.9 cm [anteroposterior (AP) x width (W) x craniocaudal (CC)] abutting the horizontal and oblique fissure (Figure 2). Positron Emission Tomography (PET)/CT showed significant uptake of fluorodeoxyglucose (FDG) at SUVmax 10.9 and evidence of mediastinal lymphadenopathy. Our respiratory colleagues underwent bronchoscopy and endobronchial ultrasound (EBUS) guided transbronchial lung biopsy (TBLB) in which the histopathological diagnosis was consistent with adenocarcinoma, American Joint Committee on Cancer (AJCC) 8th edition T2bN2M0, Stage IIIA. No mutation was detected in the Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase domain. From here onwards, this tumor is known as tumor 1 or T1.
He underwent right uniportal Video-Assisted Thoracoscopic Surgery (VATS) and right middle lobectomy in March 2018. Stations 2R, 4R, and 7 lymph nodes were harvested and sent for histopathological examination. His surgery was uncomplicated, and he had excellent postoperative recovery. He was discharged home on postoperative day 5. The final postoperative histopathological examination revealed adenocarcinoma of acinar predominant type, measuring 2.5 x 2.8 x1.8 cm (AP x W x CC). The bronchial, vascular, and nearest stapled parenchymal margins were free of tumors. All lymph nodes harvested after surgery were negative for malignant spread. The final staging for T1 was pT1cN1M0 (stage IA) according to the AJCC 8th edition staging criteria. Due to early-stage disease, the patient was not started on adjuvant therapy.
After resection of the index primary cancer, the patient was followed-up according to our institutional guidelines, which included computed tomographic scans obtained postoperatively at 6 and 12 months and then annually. The patient was apparently well until he developed episodes of hemoptysis about 13 months later (disease-free interval). Repeat CXR (Figure 3) showed small irregular opacity over the right lung. A subsequent CECT thorax (Figure 4) detected a new spiculated nodule over the anterior segment of the right upper lobe of the lung measuring 1.9 x 1.7 x 0.9 cm (AP x W x CC). (Radiological staging T1N0, Stage 1A). Endobronchial ultrasound (EBUS) guided biopsy was performed in April 2019 and demonstrated adenocarcinoma with mutation in epidermal growth factor receptor (EGFR) exon 21.p(L858R) subtypes identified by targeted molecular testing. (Here onwards is known as tumor 2, or T2). Therefore, he was diagnosed with metachronous lung adenocarcinoma, supported by the distinct expression of EGFR mutations.
A second-generation tyrosine kinase inhibitor, gefitinib, was initiated as neoadjuvant therapy. A repeat CECT thorax was performed 12 months later in March 2020, and it showed a marked response and a significant reduction in the size of the tumor over RUL (Figure 5). He underwent redo-right VATS, upper lobectomy, and lymph node resection with curative intent in August 2020. Postoperatively, the patient developed persistent air leak, which resolved after conservative management. He was discharged well. Post-operative recovery was excellent, with recovery to baseline lung function and exercise capacity.
Histopathological specimen revealed two distinct synchronous lesions, whereby lesion 2 (T2) showed adenocarcinoma with acinar predominant measuring 6 mm on the greatest dimension (ypT1a) and lesion 3 (T3), which was detected incidentally as microinvasive adenocarcinoma with the size 2mm (on glass slide measurement), ypT1mi. T2 and T3 therefore are most likely of independent clonal origin. The resected RUL specimen has a tumor-free bronchial and vascular margin. Lymph node specimen (station 10 and 11) collected showed no evidence of metastasis. The patient has continued the tyrosine kinase inhibitor Gefitinib and is currently under follow-up with the Radiotherapy Department, Sarawak General Hospital. Both the latest CXR (Figure 6) and CECT thorax (Figure 7) done in October 2020 showed no evidence of recurrence.

Figure 1: Chest radiograph in posteroanterior (PA) view showing well-defined opacity over the right middle zone.
Figure 2: A contrast-enhanced computed tomography scan in axial view demonstrates a spiculated heterogenous mass at the medial segment of the right middle lobe measuring 2.9 x 4.1 x 2.9 cm (AP x W x CC) abutting the horizontal and oblique fissures.
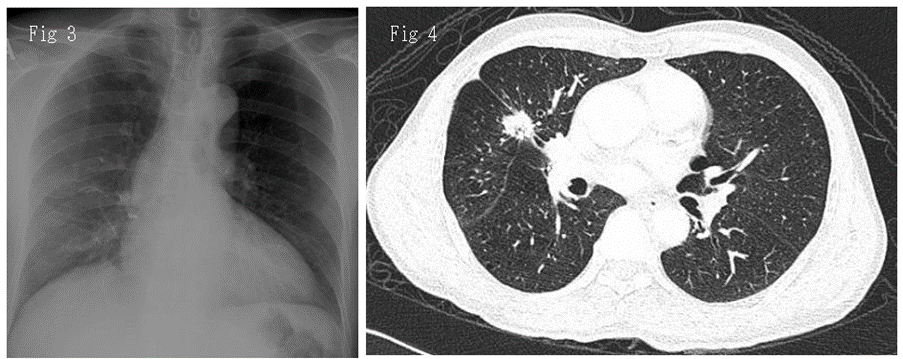
Figure 3: Chest radiograph in PA view performed 2 years after right middle lobectomy showing small irregular opacity over the right upper lung.
Figure 4: Contrasted computed tomography thoracoscopic image in axial view revealed a new speculated lesion over the right upper lobe measuring 1.9 x 1.7 x 0.9 cm (AP x W x CC).
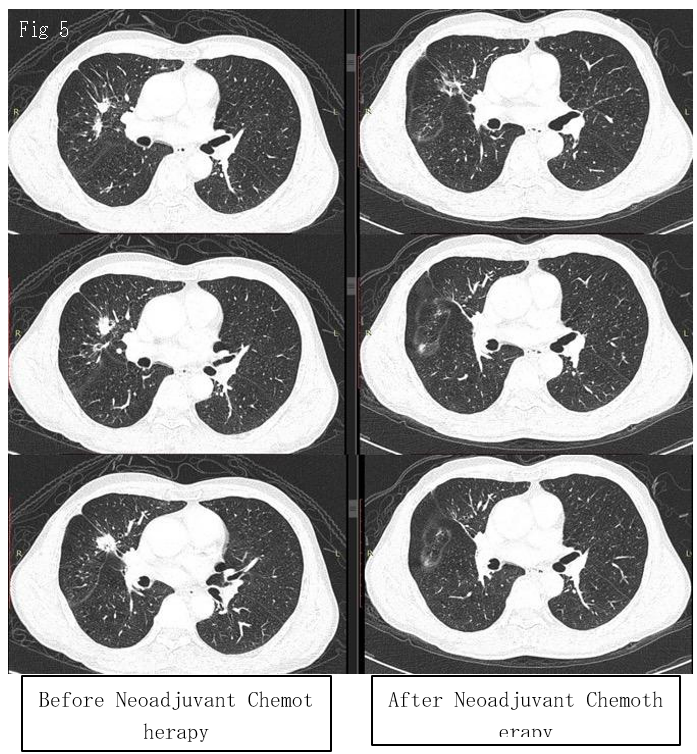
Figure 5: Comparison of nodule size before (left) and after (right) neoadjuvant tyrosine kinase inhibitor (Gefitinib) administration in contrast-enhanced computed tomography thorax taken at different level (in Axial view). This comparison demonstrated a marked radiological response of the nodule to the treatment initiated, indicating the benefits of starting TKIs upfront before lung resection in lung tumors with EGFR mutation.
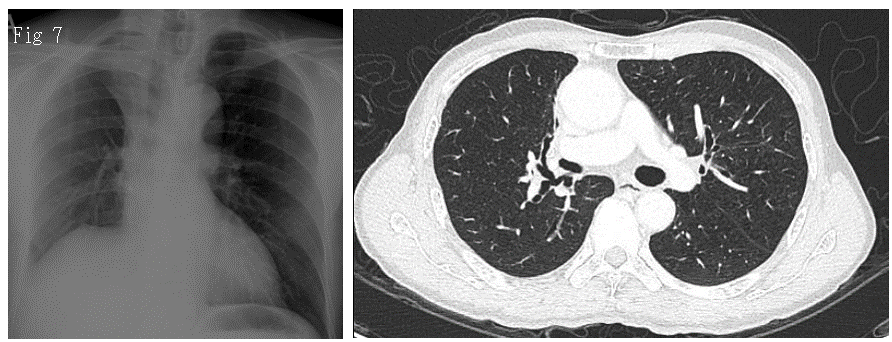
Figures 6 and 7: show the chest radiograph and CT in axial view of the patient 6 months after redo video-assisted thoracoscopic surgery (VATS), upper lobectomy. There was no recurrence detected.
Histopathological Examination:
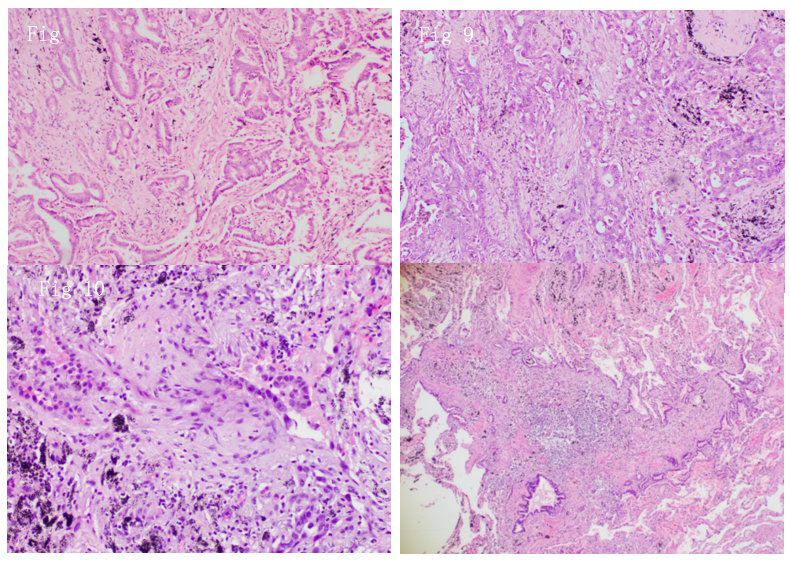
Figure 8: Right Middle Lobe Adenocarcinoma H&E:100x (2018).
Figure 9: Right Middle Lobe Adenocarcinoma H&E:100x (2018).
Figure 10: Right upper lung adenocarcinoma H&E: 200x (2020).
Figure 11: Right upper lung microadenocarcinoma H&E:40x (Incidental findings in 2020).
Both images in 2018 and 2020 showed the presence of adenocarcinoma with the same predominant histological acinar type, which was similar based on comprehensive histologic assessment. Both RML and RUL show the absence of significant nodal or systemic metastases.
Additionally, there is an incidental presence of microinvasive carcinoma (MIA) in RUL. The presence of a lepidic component at the periphery with 2-mm invasion in the acinar component also supports the concept of MLPC and/or metachronous lung cancer over IM.
Another supportive fact is the different EGFR status for both occurrence of RML (in 2018) and RUL (in 2020).
Discussion
Metachronous primary lung carcinoma was defined by Martini & Melamed as a tumor with a different histopathological nature or recurrence with a histopathological similarity with a disease-free interval of at least 2 years or a histological type that is discordant. Patients are also considered to have a metachronous second primary cancer if the tumor is associated with carcinoma in situ or arises in a different lobe without common lymphatics.
Early initiation of immunotherapy with tyrosine kinase inhibitors improves postoperative survival benefits. Prognosis is determined by the nature of the lesion and status of the expressed mutations. Unlike secondary lung metastasis, neither histologic type nor disease-free interval was of prognostic value [5]. Reports showed no difference in survival among patients diagnosed with MLC with a DFI 2 years [5,26].
In a small series of retrospective analyses using a prospectively maintained thoracic database, early tumor stage was the only significant determinant of survival after surgical treatment. of metachronous lung cancer [5]. A previous study showed a higher 5-year survival after surgical resection of metachronous stage I tumors compared with those with more locally advanced stage MLCs [26]. No significant difference in overall survival based on location of the MLC was found at 5 years [5]. It was reported that 5-year overall survival ranged from 33% to 66% after second surgery [6].
Decision on Surgical Approach
The development of a single metachronous lesion after lung cancer resection does not preclude potentially curative surgical resection in selected patients with adequate cardiopulmonary reserve and functional capacity. Operations for metachronous cancers provided survival that approximated the expected surgical intervention and should therefore be considered as a safe and effective treatment for resectable metachronous lung cancer in patients with adequate physiologic pulmonary reserve survival for lung cancer [26].
The extent of resection (lobectomy, segmentectomy, wedge resection) is often dictated by a patient’s physiologic reserve, with part of the lung segments removed prior to surgery. In fact, many centers perform sublobar resection on MLC than lobectomy [5]. The optimal management of metachronous lung cancer is affected by several factors, including associated medical comorbidities and the clinical stage of the second lung cancer.
Parenchymal preservation and morbidity associated with inadequate pulmonary reserve must be considered in patients undergoing multiple lung resections. Patients with advanced stage mMPLC or those with insufficient pulmonary reserve may not benefit from surgical resection. A Multidisciplinary Team (MDT) approach is required for surgical decisions. With MDT on board, offering different treatment modalities for all new lesions in patients with a prior history of NSCLC regardless of DFI or histologic type, would be best suited to determine long-term survival in patients with mMPLC.
The case in our report initially presented a diagnostic and therapeutic dilemma that was histologically identical and detected between an interval of two and four years. Based only on clinicopathological criteria, staging and determining whether adjuvant therapy should be initiated postoperatively are difficult. However, next-generation sequencing provided additional molecular information on these tumors and allowed us to make correct lineage calling, thus correctly identifying patients who may benefit from adjuvant therapy.
Conclusion
Diagnosis of metachronous lung cancer is challenging for clinicians in terms of surgical management. The use of molecular testing is essential to determine the lesion; thus, goal-directed therapy (immunotherapy) can be to the patient to achieve curative intent and preserve remission. It is crucial for precise staging of mMPLC, which may influence patient management and outcomes.
References
- Kan Chan Siang, John CK. A review of lung cancer research in Malaysia. Med J Malaysia. 2016; 71: 71.
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. Journal of thoracic oncology, 2015; 10(9): 1243-1260.
- Pei G, Cao S, Huang Y. Unusual metachronous lung adenocarcinomas harboring EGFR L858R/T790M mutations: A case report. Thoracic Cancer, 2020; 11(10): 3020-3023.
- Asamura H. Multiple primary cancers or multiple metastases, that is the question. Journal of Thoracic Oncology, 2010; 5(7): 930-931.
- Lee BE, Port JL, Stiles BM, Saunders J, Paul S, Lee PC, et al. TNM stage is the most important determinant of survival in metachronous lung cancer. The Annals of thoracic surgery, 2009; 88(4): 1100-1105.
- Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. The Journal of thoracic and cardiovascular surgery, 2013; 145(1): 75-82.
- Johnson BE, Cortazar P, Chute JP. Second lung cancers in patients successfully treated for lung cancer. InSeminars in oncology, 1997; 24(4): pp. 492-499.
- Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. JNCI: Journal of the National Cancer Institute, 1998; 90(18): 1335-1345.
- Martini N, Melamed MR. Multiple primary lung cancers. The Journal of thoracic and cardiovascular surgery, 1975; 70(4): 606-612.
- Rice D, Kim HW, Sabichi A, Lippman S, Lee JJ, Williams B, et al. The risk of second primary tumors after resection of stage I nonsmall cell lung cancer. The Annals of thoracic surgery, 2003; 76(4): 1001-1008.
- Loukeri AA, Kampolis CF, Ntokou A, Tsoukalas G, Syrigos K. Metachronous and synchronous primary lung cancers: diagnostic aspects, surgical treatment, and prognosis. Clinical lung cancer, 2015; 16(1): 15-23.
- Antakli T, Schaefer RF, Rutherford JE, Read RC. Second primary lung cancer. The Annals of thoracic surgery, 1995; 59(4): 863-867.
- Rubins J, Unger M, Colice GL. Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline. Chest, 2007; 132(3): 355S-367S.
- Detterbeck FC, Franklin WA, Nicholson AG, Girard N, Arenberg DA, Travis WD, et al. The IASLC Lung Cancer Staging Project: background data and proposed criteria to distinguish separate primary lung cancers from metastatic foci in patients with two lung tumors in the forthcoming eighth edition of the TNM classification for lung cancer. Journal of Thoracic Oncology, 2016; 11(5): 651-665.
- Nicholson AG, Torkko K, Viola P, Duhig E, Geisinger K, Borczuk AC, et al. Interobserver variation among pathologists and refinement of criteria in distinguishing separate primary tumors from intrapulmonary metastases in lung. Journal of Thoracic Oncology, 2018; 13(2): 205-217.
- Girard N, Deshpande C, Christopher LA, Finley D, Rusch V, William PA, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary non-small cell carcinomas from metastases. The American journal of surgical pathology, 2009; 33(12): 1752.
- Nicholson AG, Torkko K, Viola P, Duhig E, Geisinger K, Borczuk AC, et al. Interobserver variation among pathologists and refinement of criteria in distinguishing separate primary tumors from intrapulmonary metastases in lung. Journal of Thoracic Oncology, 2018; 13(2): 205-217.
- Murphy SJ, Harris FR, Kosari F, Terra SB, Nasir A, Johnson SH, et al. Using genomics to differentiate multiple primaries from metastatic lung cancer. Journal of Thoracic Oncology, 2019; 14(9): 1567-1582.
- Vignot S, Frampton GM, Soria JC, Yelensky R, Commo F, Brambilla C, et al. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol, 2013; 31(17): 2167-2172.
- de Bruin EC, McGranahan N, Mitter R, Salm M, Wedge DC, Yates L, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science, 2014; 346(6206): 251-256.
- Huang J, Behrens C, Wistuba I, Gazdar AF, Jagirdar J. Molecular analysis of synchronous and metachronous tumors of the lung: impact on management and prognosis. Annals of diagnostic pathology, 2001; 5(6): 321-329.
- Arai J, Tsuchiya T, Oikawa M, Mochinaga K, Hayashi T, Yoshiura KI, et al. Clinical and molecular analysis of synchronous double lung cancers. Lung cancer, 2012; 77(2): 281-287.
- Gazdar A, Robinson L, Oliver D, Xing C, Travis WD, Soh J, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. Journal of Thoracic Oncology, 2014; 9(4): 456-463.
- Quéré G, Descourt R, Robinet G, Autret S, Raguenes O, Fercot B, et al. Mutational status of synchronous and metachronous tumor samples in patients with metastatic non-small-cell lung cancer. BMC cancer, 2016; 16(1): 210.
- Vignot S, Soria JC. Discrepancies between primary tumor and metastasis: impact on personalized medicine. Bulletin du cancer, 2013; 100(6): 561-568.
- Battafarano RJ, Force SD, Meyers BF, Bell J, Guthrie TJ, Cooper JD, et al. Benefits of resection for metachronous lung cancer. The Journal of Thoracic and Cardiovascular Surgery, 2004; 127(3): 836-842.
- El-Sherif A, Gooding WE, Santos R, Pettiford B, Ferson PF, Fernando HC, et al. Outcomes of sublobar resection versus lobectomy for stage I non–small cell lung cancer: a 13-year analysis. The Annals of thoracic surgery, 2006; 82(2).

