Bentall Re-operation for Aortic Valve Composite Graft Endocarditis
Alen Hajdarevic1, Dragan Piljic1,*, Nail Sehic1, Mladen Predrijevac2, Mate Petricevic3, Mugdim Bajric1, Ajdin Beganovic1, Jan Kafol4 and Jus Ksela4
1University Clinical Center Tuzla, Tuzla, Bosnia and Herzegovina
2Cardivascular Hospital Center Magdalena, Krapinske Toplice, Croatia
3University Hospital Center Split, Split, Croatia
4University Clinical Center Ljubljana, Ljubljana, Slovenia
Received Date: 05/07/2024; Published Date: 11/10/2024
*Corresponding author: Dragan Piljic, M.D., Ph.D, Cardiovascular Surgery Clinic University Clinical Center Tuzla, 75000 Tuzla, Bosnia and Herzegovina
Keywords: Bentall, Endocarditis, Great saphenous vein graft, Re-operation
Introduction
Prosthetic valve endocarditis PVE accounts for 20% of all cases of endocarditis and is defined as a microbial infection that occurs on parts of a prosthetic valve or on a reconstructed natural heart valve [1]. It is the most severe form of infective endocarditis and is associated with high morbidity and mortality [2]. Early diagnosis and initiation of treatment are essential because they improve outcomes and reduce complications and mortality.
Based on the time of the disease onset, PVE is classified in two types: early and late PVE. Early PVE occurs within one year after surgery, while late PVE occurs after one year. The clinical significance of this classification lies in the microbiological profile difference between the two groups [3].
The occurrence of aortic PVE is different if aortic valve is replaced surgically (Surgical Aortic Valve Replacement (SAVR) and if transcatheter aortic valve replacement (TAVR) is performed.
In SAVR, the occurrence of PVE is 6 per 1000 cases [4]. Furthermore, the occurrence of PVE was higher in patients who had bioprosthetic SAVR than in patients with mechanical SAVR [5,6].
Late-type PVE usually occurs due to nosocomial infection when patients are admitted for other health problems or due to different exposures in outpatient settings such as transfusion centers, nursing homes, or during hemodialysis. [3].
Case Report
We present a case of a 46-year-old man with a bicuspid aortic valve and anuloaortic ectasia who initially underwent Bentall surgery. The operation and the early postoperative course were uneventful. On the seventh postoperative day, the patient was discharged from hospital. On the 15th postoperative day, on a routine control, signs of inflammation were noted in the sternal wound area, and the patient was treated with antibiotics, according to the antibiogram, on an outpatient basis. One month after the initial surgery there were no signs of inflammation on the sternal wound and the sternum was stable. The first postoperative Transthoracic Echocardiography (TTE) showed normal hemodynamic parameters over the implanted artificial valve.
Sixteen months after the initial operation, the patient complains of general weakness, elevated body temperature and fever, as well as swelling of the hands. Anemia with elevated values of inflammatory markers were recorded in the laboratory findings.
The patient was hospitalized at the Department of Cardiology. Furthermore, blood cultures were taken. And were positive for Staphylococcus warneri. Antibiotic therapy was started. The TTE findings showed perianular endocarditic collections and extremely turbulent blood flow at the level of the left ventricular outflow tract (LVOT) and around the aortic mechanical valve prosthesis. Transesophageal Echocardiography (TEE) showed dehiscence of the composit graft stent on the part of the anulus in the projection of the non-coronary sinus, with a large abscess cavity around the right coronary artery (RCA) ostium and a reoperation was indicated.
The operative procedure was performed under general anesthesia, and heart was arrested using 2000 mL of del Nido cardioplegic solution which was applied anterogradely ostially. Intraoperatively, complete destruction of the aortic anulus and composite graft dehiscence with an abscess cavity in the projection of the non-coronary sinus was noted. The ostia of the coronary arteries and the distal attachment of the conduit to the aorta were intact. The conduit was completely resected. The LVOT, aortic valve anulus, and the abscess cavity are cleaned. Left coronary artery (LCA) button was encircled with dense adhesions and therefore no significant dissection or mobilization of the ostium was possible. A conduit with a mechanical valve (St Jude aortic mech. valved graft No. 25) was implanted. The ostium of the LCA was reimplanted using the great saphenous vein graft interpositum. Cardiopulmonary bypass time was 386 minutes and cardioplegic arrest time was 256 minutes. The heart started spontaneously after warm blood reperfusion. During the operation, there were no significant disorders of acid-base status, electrolyte imbalance, arterial blood pressure, or other laboratory findings. The early postoperative course was uneventful. Postoperatively, the patient was treated with the antibiotic therapy that consisted of rifampicin, meropenem, and vancomycin. Postoperative blood cultures were sterile. At discharge, the sternum was stable, and the wound was healing properly. Postoperative TTE on the third postoperative day showed normal hemodynamic parameters over the implanted aortic valve prosthesis The patient was discharged home on the eighth postoperative day. Control TTE 15 days and two months after surgery showed normal hemodynamic parameters over the artificial valve with normal segmental and global contractility of the left ventricle, and left ventricular ejection fraction was (LVEF) 65%.
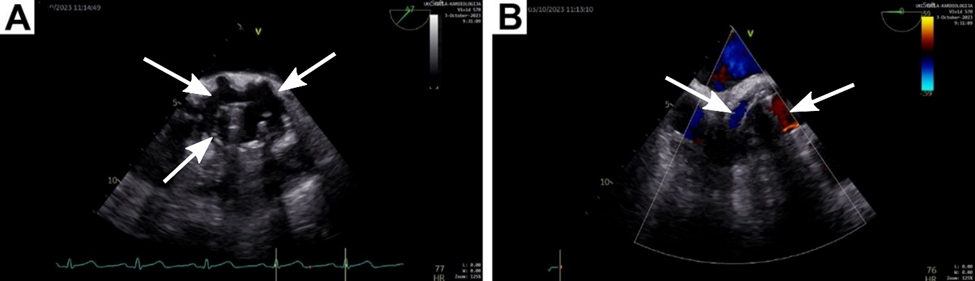
Figure 1: (A)Transthoracic echocardiography (TTE) showed a freely mobile and rocking prosthetic aortic valve, with a severe paravalvular leak causing aortic insufficiency (indicated by arrow). (B) Perigraft collection after first Bentall procedure.
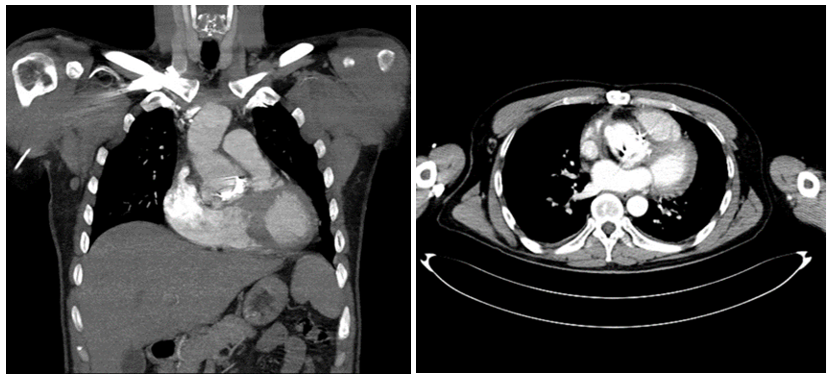
Figure 2: Preoperative CTA showing an ascending aorta prosthetic graft highly suggestive of graft infection.
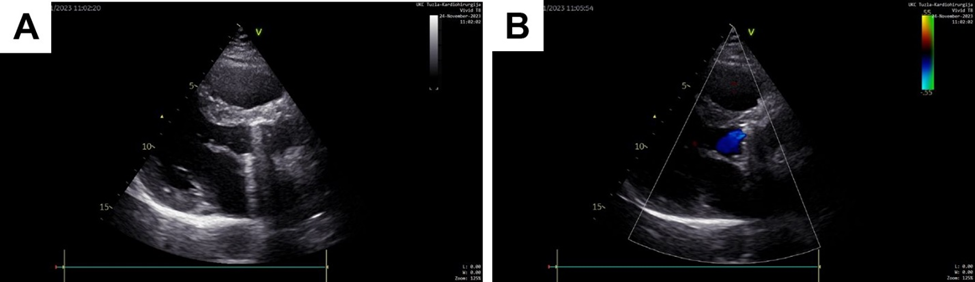
Figure 3: TTE two months after re-Bentall surgery: postoperative image of newly placed bioprosthetic aortic valve without dehiscence and paravalvular or valvular regurgitation.
Discussion
Reoperations after Bentall procedure or Ross-Konno procedure are rare and are usually performed due to PVE or the reconstructed natural heart valve. Hospital mortality after surgery was higher for (PVE) (up to 27%) when compared to Native Valve Infective Endocarditis (NVE) (16%) and cardiac device-related infective endocarditis (CDRIE) (8–15%) (7, 9). PVE surgery is indicated for mobile vegetations larger than 10 mm, heart failure, valvular dysfunction, abscess, persistent sepsis, acute renal failure, and evidence of or a high risk of embolic events [8]. Post-surgical 30-day mortality for PVE is 14–20%, and after one year, 22–36% [8]. The chosen type of prosthesis (biological or mechanical) is not related to mortality, while the aortic cross clamp time is a significant predictor of mortality [9]. A common problem is the narrowing of the coronary arteries ostia, which can occur in severe periarteritis and is very close to the endocarditis aortic prosthesis. Therefore, it may be difficult to make coronary buttons of adequate size, and coronary openings may be very fragile. In that situation, other coronary artery reimplantation techniques (e.g., Piehler's or Cabrol's method) may be useful. Considering the severe adhesive changes of the LCA ostium, a VSM graft was used in this patient, which was a practical solution.
Conclusion
Great saphenous vein graft interpositum can be used as a method of coronary button reimplantation in a setting of severe adhesive changes of coronary ostia without adding significant surgical risk.
Authors' contributions:
Concept and design of study: AH('guarantor'), DP, MPr, NS, JK
Acquisition of data or analysis and interpretation of data: DP, AH, NS, MPr, MPe, MB, AB, JKa, JKs
Drafting the article or revising it critically for important intellectual content: AH, DP,NS,MPr,JK
Final approval of the version to be published: DP, AH, NS, MPr, MPe, MB, AB, JKa, JKs
Informed consent: The patient provided written informed consent for publication of the figures.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Piper C, Körfer R, Horstkotte D. Prosthetic valve endocarditis. Heart, 2001; 85(5): 590-593.
- Glaser N, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U. Prosthetic Valve Endocarditis After Surgical Aortic Valve Replacement. Circulation, 2017; 136(3): 329-331.
- Wang A, Athan E, Pappas PA, Fowler VG, Olaison L, Paré C, et al. International Collaboration on Endocarditis-Prospective Cohort Study Investigators. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA, 2007; 297(12): 1354-1361.
- Ostergaard L, Valeur N, Ihlemann N, Bundgaard H, Gislason G, Torp-Pedersen C, et al. Incidence of infective endocarditis among patients considered at high risk. Eur Heart J, 2018; 39(7): 623-629.
- Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol, 2000; 36(4): 1152-1158.
- Glaser N, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50-69 years. Eur Heart J, 2016; 37(34): 2658-2667.
- Ortiz-Bautista C, López J, García-Granja PE, Vilacosta I, Sevilla T, Sarriá C, et al. Endocarditis infecciosa derecha en portadores de dispositivos cardiacos: perfil clínico y pronóstico, 149. Barcelona: Medicina Clínica, 2017. 10.1016/j.medcli.2017.03.055
- Pyo WK, Kim HJ, Kim JB, Jung S-H, Choo SJ, Chung CH, et al. Comparative surgical outcomes of prosthetic and native valve endocarditis. Korean Circ. J, 2021; 51: 504-514.
- Nasso G, Santarpino G, Moscarelli M, Condello I, Dell’Aquila AM, Peivandi AD, et al. Surgical treatment of valve endocarditis in high-risk patients and predictors of long-term outcomes. Sci. Rep, 2021; 11: 24223.

