Non-Hodgkin's Lymphoma of the Testis
Staouni benabdallah Y*, Sidki S, Kbirou A, Moataz A, Dakir M, Debbagh A and Aboutaieb R
The Urology Department at Ibn Rochd Hospital in Casablanca, Morocco
Received Date: 17/06/2024; Published Date: 08/10/2024
*Corresponding author: Staouni benabdallah Y, The Urology Department at Ibn Rochd Hospital in Casablanca, Morocco
Abstract
A patient was managed for primary LMNH of the testis, and retrospectively analyzed, the patient's age at diagnosis was 46 years, he benefited from an orchiectomy, and he is currently undergoing R-CHOP chemotherapy.
Primary non-Hodgkin lymphomas (NHL) of the testicle are rare, representing between 1 and 9% of testicular tumors. They are particularly common in older men (average age at diagnosis: 60 years). The most common histological type is the B type (developed from peripheral B lymphocytes). These lymphomas remain characterized by a poor prognosis.
Keywords: Non-Hodgkin testicular lymphoma; Histological diagnosis; Treatment
Introduction
Primary malignant non-Hodgkin lymphomas (NHL) of the testis are rare, accounting for 1 to 9% of testicular tumors. They are particularly common in elderly men, with an average age at diagnosis of 60 years. The most common histological subtype is the diffuse large B-cell lymphoma, originating from peripheral B lymphocytes, as classified by the WHO in 2001. These lymphomas are associated with a poor prognosis.
Observation: The patient, Mr. F.H., a 46-year-old unemployed bachelor with no significant comorbidities, presented with a notable history of toxic exposure, including active smoking at a rate of 120 packs per year and hashish use. His medical history revealed the onset of left inguinal lymphadenopathy, followed three months later by the development of a scrotal mass. A testicular ultrasound showed a large, bilobed tumor-like process in the right testicle with significant external iliac, left inguinal, and retroperitoneal lymphadenopathy. Tumor markers were negative, including LDH, AFP, and HCG. A thoracoabdominal-pelvic CT scan indicated the presence of lymphadenopathy in the para-aortic, common iliac, internal and external iliac regions bilaterally, with the largest external iliac lymph node on the left measuring 26x63 mm and a left inguinal lymph node measuring 25x37 mm. The suspicious right testicular mass measured 65x42 mm. In January 2024, the patient underwent a right orchiectomy (Figure 1). Histopathological examination (Figure 2) revealed a poorly differentiated malignant tumor measuring 6 cm in its largest dimension. Immunohistochemical studies confirmed the diagnosis of B-cell lymphoma with CD20 positivity in the tumor cells. The patient was initiated on R-CHOP chemotherapy regimen at the hematology department and is currently still undergoing treatment, radiotherapy scheduled post-chemotherapy for the contralateral testicle.
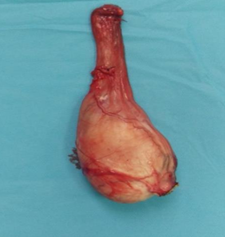
Figure 1: Right orchidectomy.
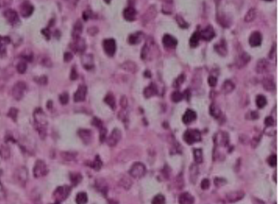
Figure 2: Infiltration of the testis by a sheet-like proliferation of large cells.
Discussion
Primary testicular lymphoma (PTL) is an uncommon manifestation of extranodal non-Hodgkin lymphoma (NHL), constituting only 1% to 2% of NHL cases and 1% to 5% of primary testicular tumors. The prevailing histological subtype is diffuse large B-cell lymphoma (DLBCL), accounting for approximately 80% to 90% of cases, predominantly affecting elderly men over 60 years of age [1,2]. However, recent literature suggests a rising incidence among younger demographics. This condition is marked by heightened aggressiveness and a substantial risk of relapse, even after a decade or more, notably affecting the contralateral testicle (in 5% to 35% of cases) and posing a risk of central nervous system (CNS) relapse (20% at five years, 35% at ten years), particularly in patients who have not undergone radiotherapy on the contralateral testicle [1,2]. PTL may occur in isolation or in association with other neoplasms or immunodeficiency states such as immunosuppressive therapy, HIV infection, or tuberculosis. Our patient, initially diagnosed with, exhibits slowly progressing local symptoms and testicular masses, with normal testicular markers. Clinical presentation typically entails painless unilateral testicular swelling and systemic B symptoms in advanced-stage disease, although bilateral involvement may occur in up to 10% of case [3]. Granulomatous orchitis, plasmacytoma, pseudolymphoma, and rhabdomyosarcoma are principal differential diagnoses [1,3-5]. PTL can infiltrate adjacent structures like the epididymis and spermatic cord, with the potential to disseminate to extranodal sites including the CNS (up to 30%), soft tissues, Waldeyer's ring (5%), skin (up to 35%), eyes, pleura and lung. Diagnostic imaging, particularly ultrasonography (US), plays a pivotal role in initial diagnosis, staging, and follow-up. Testicular masses are visualized as hypoechoic focal or diffuse lesions with increased vascularity on US, though this feature lacks specificity. CT or MRI can provide additional insights into testicular and epididymal structures, while PET/CT aids in staging, surveillance, relapse assessment, and treatment response evaluation. In our case, testicular involvement was isolated, without extratesticular spread [6]. Radical orchiectomy, serving both diagnostic and therapeutic ends, is pivotal in PTL management, albeit insufficient as standalone therapy, even in stage I patients, to secure favorable outcomes and avert distant relapses [3-5]. Multimodal therapy, encompassing orchiectomy followed by R-CHOP chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone), contralateral testicular radiotherapy (25-30 Gy in 1.5-2 Gy fractions), and CNS prophylaxis, has significantly improved the 5-year survival rate from 30% to 86.6%. Key prognostic indicators include older age, advanced stage, elevated LDH, B symptoms, and orchiectomy alone, correlating with poorer outcomes and higher mortality [3-6]. in our case, systemic chemotherapy comprising rituximab, cyclophosphamide, doxorubicin, and vincristine is being administered in the clinical hematology department. The patient is currently undergoing treatment, with radiotherapy scheduled post-chemotherapy for the contralateral testicle.
Conclusion
Primary testicular non-Hodgkin lymphomas (NHL) primarily affect individuals over the age of 60 and typically present with normal testicular markers. Surgical management through extended orchidectomy remains paramount. However, a multidisciplinary approach is essential for achieving rapid and sustained complete remission. Despite the pathology indicating infiltration of the testicle by a proliferation of large cells surrounding analyzable seminiferous tubules, autopsy studies suggest a tumor frequency nearing 19%. The age of diagnosis typically surpasses 50 years, as evidenced by our analysis with 87.5% of patients falling within this age bracket. Most testicular NHLs are localized (13–50%), consistent with our findings where seven out of eight cases presented with Ann Arbor stages IE or IIE. These figures are contingent upon the thoroughness of complementary examinations. Current staging protocols necessitate thoraco-abdomino-pelvic CT scans, with CNS imaging considered based on clinical presentation, where MRI may offer greater contribution, coupled with lumbar puncture. Recent histopathological studies employing the working formulation for clinical usage report a predominance (50–80%) of diffuse large B-cell lymphomas. Despite their traditionally grim prognosis, primary testicular NHLs treated with systemic and intrathecal adjuvant chemotherapy demonstrate favorable long-term specific survival. Nonetheless, chemotherapy-related mortality, evident in 1 case out of 8 in our series, and the inability of chemotherapy to fully shield against cerebral recurrence or contralateral testicular relapse necessitates consideration of prophylactic contralateral testicular and cerebral irradiation alongside standard adjuvant orchidectomy to mitigate relapse rates and extend remission durations.
References
- Shahab N, Doll DC. Testicular Lymphoma. Semin Oncol, 1999; 26: 259-266.
- Park BB, Kim JG, Sohn SK, Kang HJ, Lee SS, Eom HS, et al. Consideration of aggressive therapeutic strategies for primary testicular lymphoma. Am J Hematol, 2007; 82: 840-845.
- Rabii R, Mezzour M, Guessous H, Essaki H, Joual A, Rachid M, et al. Quessar A. Anurie obstructive révélatrice d’un lymphome uro-génital. Prog Urol, 2004; 14: 73-77.
- Vitolo U, Ferreri AJ, Zucca E. Primary testicular lymphoma. Critical reviews in oncology/hematology, 2008; 65(2): 183-189.
- Vitolo U, Seymour JF, Martelli M, Illerhaus G, Illidge T, Zucca E, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 2016; 27: v91-v102.
- Zucca E, Conconi A, Mughal TI, Sarris AH, Seymour JF, Vitolo U, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. Journal of Clinical Oncology, 2003; 21(1): 20-27.

