Unusual Presentation of Malignant Phyllodes Tumor with Metastasis to Axillary Nodes - A Case Report and Review of Literature
Sruthi Cheera1,*, Swathika Kannan1, Lohitha Krishna1, Sulakshanna2, Sasi Mouli1, Sandhya A3, Karthik4, Sandeep Desai5 and Srinath B1
1Department of Surgical Oncology, Sri Shankara Cancer Hospital, India
2Department of Onco Pathology, Sri Shankara Cancer Hospital, India
3Department of Medical Oncology, Sri Shankara Cancer Hospital, India
4Department of Radiation Oncology, Sri Shankara Cancer Hospital, India
5Department of Radiology, Sri Shankara Cancer Hospital, India
Received Date: 17/05/2024; Published Date: 07/10/2024
*Corresponding author: Sruthi Cheera, Department of Surgical Oncology Sri Shankara Cancer Hospital, India
Abstract
Phyllodes tumors are uncommon breast neoplasms, and distinguishing between benign and malignant variants can be challenging. Typically, metastasis occurs hematogenously, with lymphatic spread being very rare (less than 1%). In this article, we will delve into a rare case encountered by us, where a woman presented with a malignant phyllodes tumor featuring skeletal and lymph node metastasis. Following this, we will review the world literature on phyllodes tumor with axillary lymph node metastasis.
Introduction
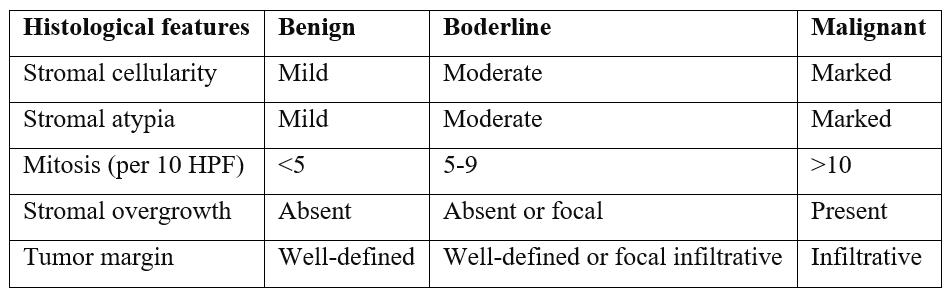
Phyllodes tumors of the breast globally account for 0.3% to 1% of breast tumors [1]. The term 'cystosarcoma phyllodes' was first coined by Johannes Muller in 1838, deriving its name from the Greek words 'sarcoma,' meaning flesh-appearing tumor, and 'phyllon,' meaning leaf-like [2]. The World Health Organization (WHO) classifies this tumor into benign, borderline, and malignant categories based on histological features such as stromal cellularity, stromal overgrowth, stromal atypia, mitoses/high power field, and tumor margin [7]. Typically, they are commonly found in middle-aged women aged between 35 and 55 years [3]. The biological behavior of the phyllodes tumor is determined by its mesenchymal component.
Malignant phyllodes tumors often exhibit an overgrowth by the malignant mesenchymal component, resulting in a reduction of the epithelial component, including the distinctive leaf-like pattern and the emergence of a spindle cell neoplasm [4]. Clinically and radiologically, diagnosing malignant phyllode tumors can be challenging, as they share similarities with benign lesions such as fibroadenomas and benign phyllodes. Approximately 10-15% of phyllodes tumors are malignant, and among these, 9-27% may develop metastatic disease [5]. Since most malignant phyllodes tumors metastasize via the bloodstream, nodal involvement is rare, and axillary lymph node dissection is not routinely indicated [6]. Following surgical excision, the majority of these tumors require adjuvant treatment. In this context, we present a case and conduct a literature review on malignant phyllodes with axillary lymph node metastasis.
Case Presentation
A 66-year-old post-menopausal female, a mother of two, presented with upper back pain, difficulty walking, and left leg weakness, prompting an evaluation elsewhere. MRI of the spine revealed a hyperintense lesion at the D3 level involving the right pedicle, rib head, and transverse process, accompanied by an enhancing soft tissue component measuring 29x30x37 mm, causing cord compression.
During the evaluation, it was discovered that she had a long-standing left breast lump. Physical examination revealed a lobulated lump measuring 10 x 8 cm, involving the central quadrant of the left breast with cystic-solid consistency. The overlying skin was erythematous with dilated veins, and the normal nipple-areola complex was observable. Lymph nodes were found to be enlarged and mobile in the left axilla, while examination of the contralateral breast, axilla, and neck revealed no abnormalities. A core biopsy from the left breast lump suggested a benign phyllodes tumor.
A PET CT SCAN further revealed an 18 F FDG avid, partially necrotic, large lobulated mass in the left breast, likely representing a phyllodes tumor. Additionally, an 18F FDG avid lytic lesion was identified in the T3 vertebra with an extradural soft tissue component, indicating a metastatic lesion.
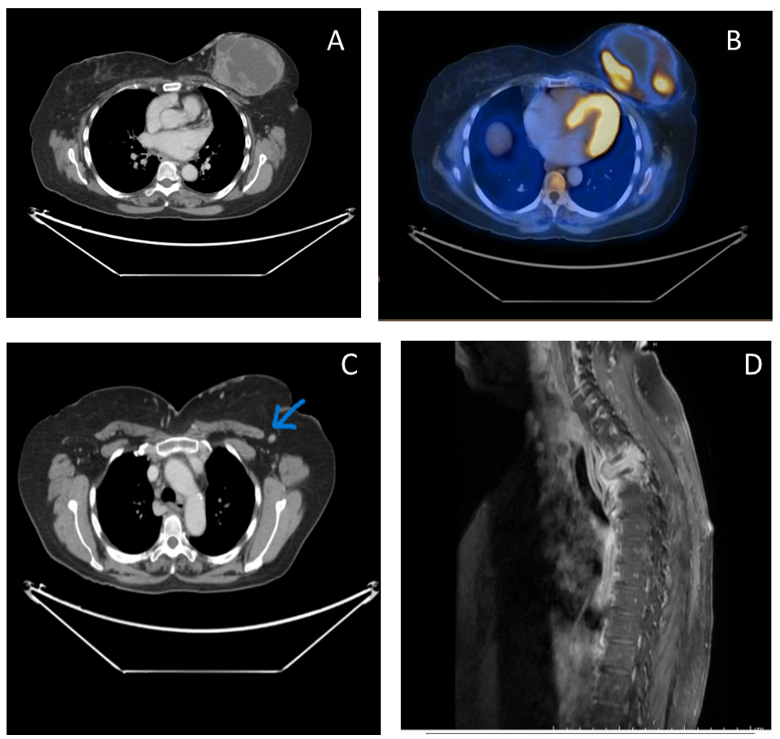
Figure A, B, C - show CT and PET CT images demonstrating a tracer avid solid cystic lesion in the left breast with metastatic left axillary lymph node.
Figure D – MRI showing pathological fracture of D3 vertebral body with epidural soft tissue component causing canal compromise suggestive of metastasis.
Subsequently, the patient underwent D1-D6 instrumental fusion, posterior decompression with D3 laminectomy, and gross tumor total excision with iliac graft placement. The histopathology report of the D3 lesion indicated a spindle cell neoplasm. Immunohistochemistry (IHC) results suggested features of undifferentiated high-grade sarcoma, as the tumor exhibited positivity for SMA, CD99, and BCL2, while being negative for desmin, H-caldesmon, S-100, CD34, EMA, pan-cytokeratin, and STAT6. The proliferation index was measured at around 80–85%. She presented to our institution with the aforementioned reports. Sarcomas conventionally requires a higher dose of radiation for disease control to a range of 60 -70 Gy in 2 Gy per fraction. However, the spinal cord tolerance is only 45-50 Gy maximum. As the spinal lesion is the only site of disease, to achieve maximum tumoricidal dose with minimum normal tissue damage, the Stereotactic body radiotherapy technique was utilized and 40 Gy in 8 fractions was delivered on alternate days.
In the multidisciplinary discussion, it was decided to proceed with breast surgery. This decision was influenced by the understanding that the initial biopsy from the breast might not represent the entire mass, and furthermore, there was an associated sarcoma.
The second stage involved a left total mastectomy with axillary lymph node sampling due to intraoperatively noted enlarged axillary lymph nodes. Histopathology results indicated a malignant phyllodes tumor with marked stromal cellularity, stromal atypia, stomal overgrowth, and 26 mitoses per 10 high power fields. Notably, 1 out of 3 lymph nodes showed involvement. Considering the diagnosis of malignant phyllodes with vertebral and lymph node metastasis, the treatment focused on disease control. Subsequently, the patient received 3 cycles of doxorubicin and ifosfamide once every 3 weeks.
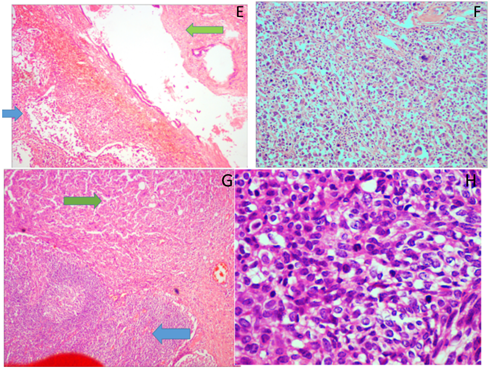
Figure E: Breast parenchyma displaying benign and malignant stromal components (blue arrow – malignant component; green arrow – benign component); H&E, 10X.
Figure F: Sections from breast parenchyma displaying highly pleomorphic spindled tumor cells in sheets (H & E, 10X).
Figure G: Sections from the axillary lymph node displaying metastatic tumor (blue arrow – lymph node; green arrow – tumor); H & E, 10X.
Figure H: Sections from the spinal lesion displaying pleomorphic spindled to oval cells arranged in fascicles (H & E, 10X & 40X).
Discussion
Phyllodes tumors of the breast are rare fibroepithelial neoplasms with a wide range of clinical behaviors. Malignant phyllodes tumors are characterized by marked nuclear pleomorphism of stromal cells, stromal overgrowth (defined by the absence of the epithelial component in one low-power microscopic field), diffuse stromal cellularity with increased mitotic activity (> 10 per 10 high-power fields), and infiltrative borders. In 2012, the 4th edition of the WHO Breast Tumor Classification introduced a table listing the histological criteria for phyllodes tumors [7].
Phyllodes tumors typically manifest as rapidly growing breast lumps, with other symptoms and signs that are non-specific, making clinical diagnosis challenging. Distinguishing between Phyllodes Tumors (PT) and fibroadenomas through ultrasound (US) and mammography can be difficult. On mammography, a PT typically presents as a well-circumscribed, hyperdense or isodense, round or oval mass. Conversely, significant sonographic features favoring the diagnosis of Phyllodes Tumors (PT) over fibroadenomas include a lobulated shape, a heterogeneous internal echo pattern, and the absence of microcalcifications. Importantly, neither sonography nor mammography can reliably distinguish between malignant and benign PTs [8,9]. While FNAC is not a reliable method to distinguish a fibroadenoma from a phyllodes tumor, core needle biopsy has been proposed as a more effective approach for preoperative diagnosis. Nevertheless, the definitive diagnosis should ultimately rely on the pathological examination of surgical specimens [10].
Immunohistochemistry reveals distinctive features, with the expression of p53, Ki-67, CD117, EGFR, p16, and VEGF showing the highest levels in malignant phyllodes tumors and the lowest positivity in benign cases [11]. Some authors have delved into the genetic landscape of fibroepithelial lesions, revealing a similarity in the profiles of benign phyllodes tumors to fibroadenomas. In contrast, malignant phyllodes tumors were identified as claudin-low and basal-like [12]. Genome-wide analyses involving DNA copy number variations and genomic sequencing have unveiled notable amplifications and deletions. In addition to loss-of-function mutations in TP53, deleterious mutations in RB1 and NF1, mutations in PIK3CA and ERBB4, and high-level copy number variations of EGFR have been detected in borderline/malignant tumors [13,14].
Hawkins et al. and Kessinger et al. established a robust correlation between stromal overgrowth and metastasis, noting that all metastatic lesions contained only stromal elements. Phyllodes tumors inherently harbor recurrence and/or metastatic potential, varying based on histologic grade. Clinical behavior may not always align with histologic appearance, as both malignant and borderline tumors exhibit metastatic capabilities [15,16]. Distant metastasis is observed in 25% of malignant phyllodes tumors, compared to 10% in all phyllodes tumors. Interestingly, 3–12% of metastatic cases emerge at the time of presentation or as late as more than 10 years later [17]. The most frequent sites for metastasis in malignant phyllodes tumors are the lung (66%), bones (28%), brain (9%), and liver [18,19]. Rare instances of distant metastasis involve the adrenal glands, kidney, skin, ovary, heart, pleura, oral cavity, duodenum, pancreas, tonsillar, and para-aortic nodes. Similar to other sarcomas, malignant phyllodes tumors primarily metastasize hematogenously, with lymphatic spread being exceptionally rare [20]. As per NCCN guidelines, the recommended treatment for phyllodes tumors, encompassing benign, borderline, and malignant subtypes, involves local surgical excision with tumor-free margins of 1 cm or greater. Lumpectomy is the preferred surgical approach, with total mastectomy deemed necessary only if lumpectomy cannot achieve negative margins. Some literature also describes a more radical approach, involving excision of the tumor with a 2-cm margin, encompassing overlying skin and a portion of the underlying pectoralis major muscle, aiming to mitigate local recurrence [36].
Due to the rare occurrence of lymph node metastasis in malignant phyllodes tumors, routine axillary lymph node dissection is not a common practice. In some cases, particularly in patients undergoing total mastectomy, sentinel lymph node biopsy or axillary sampling might be performed. According to the literature, the reported incidence of axillary lymphadenopathy is approximately 20–25% [21]. Axillary lymphadenopathy is frequently indicative of an infected necrotic tumor rather than metastasis [6]. However, in some instances, malignant transformation within the epithelial component of phyllodes tumors can occur, leading to the development of an associated invasive or in-situ component (reported at 1.1 to 6%) and, consequently, metastatic axillary lymph nodes [37].
Studies conducted by Bennett et al., Ramakant P et al., Chaney AW et al., and Narayanakar RP et al., involving clinicopathological data and management of phyllodes tumors, included 30, 160, 101, and 162 patients, respectively. In these studies, lymphatic spread was also evaluated, and they showed no evidence of nodal metastasis [20-22].
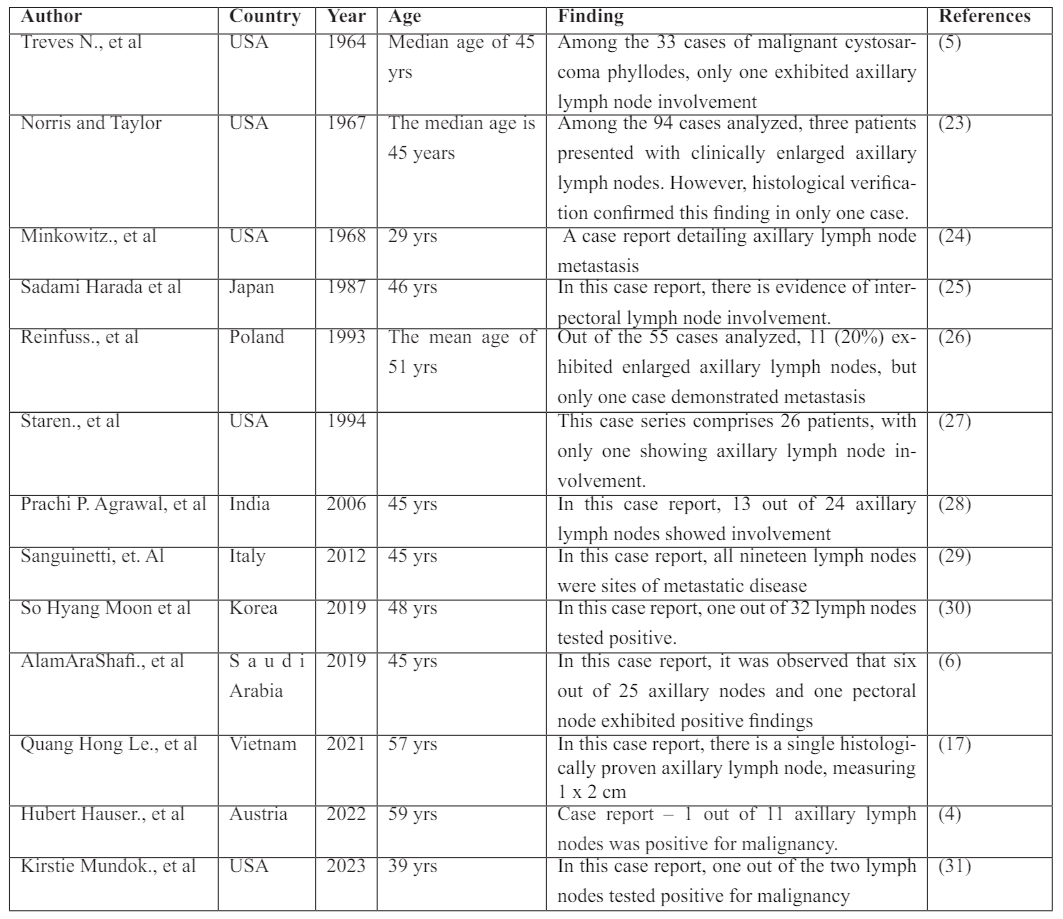
In the literature, only a sparse number of cases featuring malignant phyllodes (without an associated invasive component) with involvement of metastatic lymph nodes have been reported.
Benign phyllodes tumors typically do not necessitate further adjuvant treatment after margin-negative surgical resection [32]. For borderline and malignant phyllodes tumors, adjuvant radiotherapy is recommended, irrespective of the type of surgery performed [33]. Adjuvant chemotherapy is considered for high-risk individuals, including those with tumors larger than 5 cm, stromal overgrowth, and positive surgical margins where re-excision is not feasible [34,35]. The European Society for Medical Oncology (ESMO) guidelines align with criteria similar to those for extremity sarcomas.
In the first-line therapy, a combination of Anthracycline and Ifosfamide (AI) may be more effective, especially for patients with a performance status suitable for combination therapy. Upon progression, in addition to a new line of chemotherapy, pazopanib or trabectedin are reasonable options according to NCCN guidelines.
Conclusion
Malignant phyllodes tumor of the breast is known for its unpredictable and sometimes highly aggressive nature. Reported cases of phyllodes with lymph node involvement remain scarce in the literature. Consideration for axillary lymph node biopsy or sampling is warranted in selective cases, particularly those with large tumor sizes and suspicious locoregional nodes, especially in patients undergoing total mastectomy.
References
- Abe H, Teramoto A, Takei Y, Tanaka Y, Yoneda G. Malignant phyllodes tumor of the breast with rapid progression: a case report. Surgical Case Reports, 2020; 6(1): 1-5.
- McGregor GI, Knowling MA, Este FA. Sarcoma and cystosarcoma phyllodes tumors of the breast—a retrospective review of 58 cases. The American journal of surgery, 1994; 167(5): 477-480.
- Testori A, Meroni S, Errico V, Travaglini R, Voulaz E, Alloisio M. Huge malignant phyllodes breast tumor: a real entity in a new era of early breast cancer. World Journal of Surgical Oncology, 2015; 13: 1-4.
- Hauser H, Hammer R, Schöllnast H, Humer-Fuchs U, Kriegl D, Fuchsjäger M, et al. Malignant phyllodes tumor of the breast with axillary lymph node metastasis: case report and review of the literature. European Surgery, 2022; 54(3): 156-162.
- Treves N. A study of cystosarcoma phyllodes. Annals of the New York Academy of Sciences, 1964; 114(2): 922-936.
- Shafi AA, Al Harthi B, Riaz MM, AlBagir A. Gaint phyllodes tumor with axillary & interpectoral lymph node metastasis; a rare presentation. International Journal of Surgery Case Reports, 2020; 66: 350-355.
- Iqbal S, Iqba J, Nowshad N, Mohammad K. A Malignant phyllodes Tumor of the Breast; Presentation of an Uncommon Case and Review of the Literature. Archives of Breast Cancer, 2020; 93-96.
- Papas Y, Asmar AE, Ghandour F, Hajj I. Malignant phyllodes tumors of the breast: A comprehensive literature review. The Breast Journal, 2020; 26(2): 240-244.
- Chao TC, Lo YF, Chen SC, Chen MF. Sonographic features of phyllodes tumors of the breast. Ultrasound in Obstetrics and Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology, 2002; 20(1): 64-71.
- Komenaka IK, El-Tamer M, Pile-Spellman E, Hibshoosh H. Core needle biopsy as a diagnostic tool to differentiate phyllodes tumor from fibroadenoma. Archives of surgery, 2003; 138(9): 987-990.
- Karim RZ, Gerega SK, Yang YH, Spillane A, Carmalt H, Scolyer RA, et al. p16 and pRb immunohistochemical expression increases with increasing tumour grade in mammary phyllodes tumours. Histopathology, 2010; 56(7): 868-875.
- Md Nasir ND, Ng CC, Rajasegaran V, Wong SF, Liu W, Ng GX, et al. Genomic characterisation of breast fibroepithelial lesions in an international cohort. The Journal of Pathology, 2019; 249(4): 447-460.
- Kim JY, Yu JH, Nam SJ, Kim SW, Lee SK, Park WY, et al. Genetic and clinical characteristics of phyllodes tumors of the breast. Translational Oncology, 2018; 11(1): 18-23.
- Ng CC, Nasir ND, Loke BN, Tay TK, Thike AA, Rajasegaran V, et al. Genetic differences between benign phyllodes tumors and fibroadenomas revealed through targeted next generation sequencing. Modern Pathology, 2021; 34(7): 1320-1332.
- Tan PH, Jayabaskar T, Chuah KL, Lee HY, Tan Y, Hilmy M, et al. Phyllodes tumors of the breast: the role of pathologic parameters. American Journal of Clinical Pathology, 2005; 123(4): 529-540.
- Hawkins RE, Schofield JB, Fisher C, Wiltshaw E, McKinna JA. The clinical and histologic criteria that predict metastases from cystosarcoma phyllodes. Cancer, 1992; 69(1): 141-147.
- Le QH, Mai VT. Malignant phyllodes tumor with synchronous metastases to axillary lymph nodes, lung at the presentation: a case report and literature review. Journal of Surgical Case Reports, 2021; 2021(7): rjab302.
- Ganesh V, Lee J, Wan BA, Rakovitch E, Vesprini D, Slodkowska E, et al. Palliative treatment of metastatic phyllodes tumors: a case series. AME Case Reports, 2017; 1.
- Mishra SP, Tiwary SK, Mishra M, Khanna AK. Phyllodes tumor of breast: a review article. International Scholarly Research Notices, 2013; 2013.
- Narayanakar RP, Gangaiah DM, Althaf S, Dev K, Kurpad V, Gurawalia J. Cystosarcoma phyllodes: Pathological enigma: A retrospective review of 162 cases. Indian Journal of Cancer, 2015; 52(3): 365-368.
- Bennett IC, Khan A, De Freitas R, Chaudary MA, Millis RR. Phyllodes tumours: a clinicopathological review of 30 cases. Aust N Z J Surg, 1992; 62(8): 628-633. doi: 10.1111/j.1445-2197.1992.tb07534.x.
- Ramakant P, Chakravarthy S, Cherian JA, Abraham DT, Paul MJ. Challenges in management of phyllodes tumors of the breast: a retrospective analysis of 150 patients. Indian Journal of Cancer, 2013; 50(4): 345-348.
- Norris HJ, Taylor HB. Relationship of histologic features to behavior of cystosarcoma phyllodes. Analysis of ninety‐four cases. Cancer, 1967; 20(12): 2090-2099.
- Minkowitz S, Zeichner M, di Maio V, Nicastri AD. Cystosarcoma phyllodes: A unique case with multiple unilateral lesions and ipsilateral axilary metastasis. The Journal of Pathology and Bacteriology, 1968; 96(2): 514-517.
- Harada S, Fujiwara H, Hisatsugu T, Sugihara H. Malignant cystosarcoma phyllodes with lymph node metastasis—A case report—. The Japanese journal of surgery, 1987; 17: 174-177.
- Reinfuss M, Mituś J, Smolak K, Stelmach A. Malignant phyllodes tumours of the breast. A clinical and pathological analysis of 55 cases. European Journal of Cancer, 1993; 29(9): 1252-1256.
- Staren ED, Lynch G, Boyle C, Witt TR, Bines SD. Malignant cystosarcoma phyllodes. The American Surgeon, 1994; 60(8): 583-585.
- Agrawal PP, Mohanta PK, Singh K, Bahadur AK. Cystosarcoma phyllodes with lymph node metastasis. Community Oncology, 2006; 1(3): 44-46.
- Sanguinetti A, Bistoni G, Calzolari F, Lucchini R, Monacelli M, Triola R, et al. Cystosarcoma phyllodes with muscular and lymph node metastasis. Ann. Ital. Chir, 2012; 83: 331-336.
- Moon SH, Jung JH, Lee J, Kim WW, Park HY, Lee JW, et al. Complete remission of giant malignant phyllodes tumor with lung metastasis: A case report. Medicine, 2019; 98(22).
- Mundok K, Saldana D, Mundok KA. A Large Phyllodes Tumor with Axillary Lymph Node Involvement: A Case Report. Cureus, 2023; 15(6).
- Boutrus RR, Khair S, Abdelazim Y, Nasr S, Ibraheem MH, Farahat A, et al. Phyllodes tumors of the breast: Adjuvant radiation therapy revisited. The Breast, 2021; 58: 1-5.
- Chao X, Chen K, Zeng J, Bi Z, Guo M, Chen Y, et al. Adjuvant radiotherapy and chemotherapy for patients with breast phyllodes tumors: a systematic review and meta-analysis. BMC cancer, 2019; 19: 1-7.
- Parkes AM, Patel S, Leung CH, Lin HY, Conley AP, Somaiah N, et al. Systemic therapy regimen outcomes in metastatic phyllodes tumors of the breast.
- Fede ÂB, Pereira Souza R, Doi M, De Brot M, Aparecida Bueno de Toledo Osorio C, Rocha Melo Gondim G, et al. Malignant phyllodes tumor of the breast: a practice review. Clinics and Practice, 2021; 11(2): 205-215.
- Kataria K, Dhar A, Ranjan P, Kumar A, Islam S, Srivastava A. Radical mastectomy SANS axillary lymph node dissection for large phyllodes tumor: a guarantee against recurrence. Indian Journal of Surgery, 2019; 81: 520-524.
- Gemci ÖD, Altınay S, Tartar Rİ, Ferahman S. Unexpectedly High Coexistence Rate of In Situ/Invasive Carcinoma in Phyllodes Tumors. 10-Year Retrospective and Review Study. European Journal of Breast Health, 2022; 18(4): 343.

