Hypospadias: Evaluation, Management, and Future Perspectives
Ana I Gonzalez1,*, Jonathan Valdivia1, Yesenia Brito1, Henry C Valdivia1 and Mohamed N Jabri2
1Saint George’s University School of Medicine, Grenada
2Department of Pediatrics, Alexian Brothers Hospital, Chicago, USA
Received Date: 14/06/2024; Published Date: 04/10/2024
*Corresponding author: Ana I Gonzalez, Saint George’s University School of Medicine, Grenada
Abstract
Hypospadias is a congenital malformation with malpositioning of the external urethral meatus in males and females. It typically presents with abnormal foreskin, ventral penile curvature, and two meatal openings. The level of severity dictates the management of hypospadias. In mild cases, surgery is not required; however, in significant displacement or symptomatic patients, treatment varies by the age of the child.
We present a case of a 1-day-old newborn male with hypospadias. Treatment included monitoring and referral to a urologist since the patient presented early in life.
Categories: Pediatrics; Radiology; Urology
Keywords: Hypospadias; Glandular hypospadias; Penoscrotal hypospadias; Distal hypospadias
Introduction
Hypospadias refers to the abnormal opening of the urethra in the ventral region of the penis in males or through the vaginal wall in females [1]. It occurs in up to 4.1/1000 births due to a defect in the formation of the urogenital folds during the second trimester [2]. Hypospadias can be classified into three categories based on their location along the penile shaft. Glandular hypospadias is near the head of the penis and constitute 50% of the cases. Distal hypospadias is close to the midshaft, and penoscrotal hypospadias, as the name implies, are proximal to the scrotal region [1]. In females, hypospadias is rare and difficult to detect, often overlooked until the teenage years when the female starts complaining of recurrent urinary tract infections or incontinence [3]. Antenatal ultrasound can detect hypospadias, especially in males with associated chordee or incomplete prepuce [2].
Abnormal levels of androgens and estrogens have been linked to the development of hypospadias, as genital development is a heavily hormone-driven process [4]. Endocrine-disrupting chemicals, such as phytoestrogens, pesticides, and pharmaceuticals, have been shown to disrupt Leydig and Sertoli cell activity and can lead to the formation of hypospadias [3][4]. Androgen stimulation therapy is used preoperatively to stimulate the glans size and stopped 1-2 months before surgical intervention to prevent postoperative complications such as wound dehiscence [5].
The definitive management of hypospadias is surgical intervention or urethroplasty before the second year of life [5]. The prognosis is usually favorable, especially if the defect is detected and corrected in early life [1]. Penoscrotal hypospadias is often the most challenging to repair and will often require skin grafts. Therefore, it is recommended to avoid circumcision in all cases of penile abnormalities since the foreskin could be utilized as a graft [5]. The ventral penile curvature is a preoperative measure that can guide the surgical approach of a surgeon, as it will need to be corrected if the angle goes above 15 degrees [5]. Complications are common regarding hypospadias repairs and might cause patients to require multiple surgeries to repair dehiscence, fistulas, strictures, or the persistence of penile curvatures [6].
Overall, the management of hypospadias varies depending on the severity of the defect, the sex of the patient, and overall healthcare goals. It is essential to consider the factors that might increase postoperative complications and the psychosocial impact these surgeries could have on the patient during adolescence. Achieving normal anatomy should not compromise functionality or quality of life. In clinical trials, men with a history of hypospadias repair tend to report a higher frequency of urinary symptoms, lower sexual satisfaction, and higher rates of discontent with penile appearance when compared to men without a history of hypospadias [7]. As surgical approaches evolve, we hope that dissatisfaction rates post-repair can be eliminated.
Case Presentation
Maternal Information: The mother is a primigravida, G1/P0, with a blood type of O positive. Her prenatal screening results were notable for negative Group B Streptococcus (GBS) status, negative Rapid Plasma Reagin (RPR) status, immunity to Rubella, and negative results for Hepatitis B, HIV, herpes, chlamydia, and gonorrhea. The third-trimester screening for HIV was also negative.
Labor and Delivery: Labor was induced at 39 weeks and four days of gestation through artificial rupture of membranes and oxytocin infusion, proceeding without complications. Fetal well-being was monitored externally using a fetal monitor. The labor progressed through a 4-hour first stage, a 49-minute second stage, and concluded with a 3-minute third stage, totaling approximately 4.87 hours. The delivery was vaginal.
Neonatal Presentation: The neonate was born with a birth weight of 6lb 10oz. Apgar scores at 1 and 5 minutes were 9, reflecting a healthy neonate. Initial vitals showed a temperature range between 97.9 °F and 98.7 °F, a heart rate of 120 beats per minute, and a respiratory rate of 40 breaths per minute.
Physical Examination and Diagnosis: A comprehensive physical examination revealed a well-appearing infant with normal skin color and no apparent lesions. All systems, including head, eyes, ears, nose, throat, thorax, lungs, heart, umbilicus, abdomen, femoral pulses, anus, trunk, spine, extremities, and neurologic reflexes, were within normal limits. However, a genital examination identified the presence of hypospadias in an otherwise normally descended male genitalia (Figure 1).
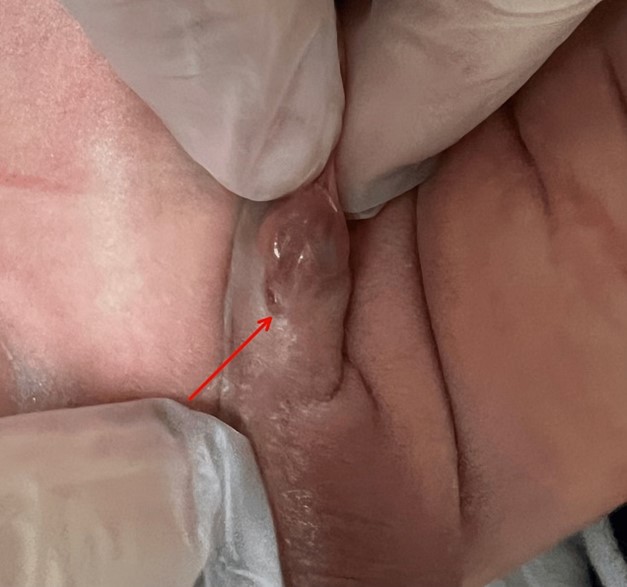
Figure 1: Abnormal placement of the opening of the urethra on the ventral side of the penis rather than at the tip.
Transcutaneous bilirubin levels were assessed (refer to Table 1, "Bilirubin Test Results"), confirming that the neonate's bilirubin levels were within a normal range.
Table 1: Bilirubin Test Results.

Management and Referral: The diagnosis encompasses a full-term baby boy with neonatal hypospadias. The management plan includes further evaluation and an early referral to a pediatric urologist for specialized care.
Discussion
Hypospadias is a congenital anomaly that typically occurs in males. It typically presents with a proximal displacement of the urethral opening, penile curvature, and a ventrally deficient hooded foreskin [8]. Hypospadias is classified based on the anatomical location of the displaced urethral orifice into distal-anterior (located on the glans or distal shaft and the most common type), intermediate-middle (penile), and proximal-posterior (penoscrotal, scrotal, perineal) [9]. Distal-anterior hypospadias is the most prevalent type among these classifications [9]. Hypospadias is diagnosed clinically. In patients who present with hypospadias, it is important to rule out sexual differentiation causes by performing an endocrinological evaluation; nonetheless, it should be pointed out that endocrinological evaluation is not mandatory in distal hypospadias. These patients typically present with concomitant ambiguous genitalia, cryptorchidism, male subfertility, and testicular cancer [10].
Recent studies suggest that hypospadias, along with cryptorchidism, poor semen quality, and testicular germ cell cancer, may be part of a broader condition known as testicular dysgenesis syndrome (TDS) [11]. TDS is thought to arise from disrupted testicular development due to genetic, environmental, and lifestyle factors, including exposure to endocrine-disrupting chemicals (EDCs) and intrauterine growth disorders [11]. The syndrome highlights the impact of both prenatal and postnatal factors on male reproductive health. Research underscores the importance of understanding these complex interactions to address the increasing incidence of these reproductive disorders, particularly in highly industrialized regions [11]. Prioritizing research in reproductive physiology and pathophysiology is crucial to developing effective prevention and treatment strategies for TDS [11].
Annually, the prevalence of hypospadias internationally is 20.9 per 10,000 births, with the majority of cases in males compared to females. Patients with a positive family history of hypospadias are more likely to express it if the malformation affected first-, second-, or third-degree relatives [12]. The two most common syndromes linked with hypospadias include Wilms’ tumor, aniridia, genitourinary malformations, range of developmental delays (WAGR), and the Denys-Drash syndrome. Incidence is shown to have increased in children with small gestational age and monochorionic twins. Other prenatal factors that should be monitored to prevent hypospadias include maternal hypertension, oligohydramnios, and premature delivery [13].
Hypospadias treatment varies based on severity and symptoms. The current guidelines for hypospadia repair are somewhere between 6 and 18 months of age. Surgery is usually the treatment of choice unless it is a very mild case. An essential step in management is determining if significant curvature persists after ventral dissection. It was determined that dorsal plication was applicable only in minor degrees of curvature [14]. One important benefit to performing surgery before 18 months of age is the lack of genital awareness in the patient. Studies have shown that adolescents who do not recall the surgery are more likely to have a positive body image throughout life [15]. Post-surgery, most boys are seen twice in the first three months and are typically monitored into puberty. Once toilet trained, he is asked to urinate in the office to evaluate for a strong urinary stream.
Complications that can arise from the surgical repair include urethral fistulas, meatal stenosis (which would require a meatotomy), a urethral diverticulum, and frequent urinary tract infections (UTIs) [16]. In proximal and complex hypospadias repair, an ultrasound or an endoscopy of the urethra during the repair is suggested to rule out the presence of urethral anomalies. If these anomalies go undiscovered, they predispose the patient to more frequent UTIs [17]. If surgery is deferred, then the patient is most likely to experience sexual dysfunction and difficulty urinating while standing [18].
Our case centers on a neonate diagnosed with hypospadias, prompting the need for further evaluation by a urologist. This evaluation aims to identify the most effective management approach, ultimately aiming to prevent long-term complications. Looking ahead, we would like to leverage advancing technology to help us administer proper treatment with minimal post-surgical complications in the future.
Conclusion
Hypospadias, arising from congenital malformations, presents a potential threat to urinary function if left unaddressed, leading to enduring complications. Surgical repair, while essential, introduces its own set of challenges in the form of urethral fistulas, meatal stenosis, urethral diverticulum, and an elevated risk of urinary tract infections in the long term. To navigate these complexities, timely referral to a urologist is vital for determining optimal management. Surgical intervention or urethroplasty emerges as the recommended path for definitive treatment, particularly for patients before the age of two. Postoperative vigilance through close follow-up becomes imperative, serving as a preventive measure against further complications in the patient's journey to recovery.
Informed Consent: A signed statement of informed consent was obtained for publication of the details of this case.
Competing Interests: None
Grant Information: None
Author Contributions:
Ana I Gonzalez: Concept and design of study, acquisition of data, drafting article, revising article. Guarantor Author.
Jonathan Valdivia: Concept and Design of study, drafting article intellectual content, revising article.
Yesenia Brito: Acquisition of data, drafting article, revising article.
Henry C Valdivia: Acquisition of data, drafting article, revising article.
Mohamed N Jabri: Final Approval, Intellectual Content, revising article
References
- Teresa NS: American Journal of Obstetrics & Gynecology, 2021; 225(5): B18-B20. 10.1016/j.ajog.2021.06.045
- Li X, Liu A, Zhang Z, et al. Prenatal diagnosis of hypospadias with 2-dimensional and 3-dimensional ultrasonography. Sci Rep, 2019; 17: 8662. 1038/s41598-019-45221-z
- Bavadiya G, Shah C, Sarkar KK, et al. Unusual presentation of female bladder outlet obstruction- female hypospadias with urethral stenosis. Urol Case Rep, 2020; 8: 101243. 1016/j.eucr.2020.101243
- Mattiske DM, Pask AJ. Endocrine disrupting chemicals in the pathogenesis of hypospadias; developmental and toxicological perspectives. Curr Res Toxicol, 2021; 1: 179-191. 1016/j.crtox.2021.03.004
- Castagnetti M, El-Ghoneimi A. Surgical management of primary severe hypospadias in children: an update focusing on penile curvature. Nature Reviews Urology, 2022; 19(3): 147-160. 1038/s41585-021-00555-0
- Abbas T, Fernandez N, Harper L. Recent advances in hypospadiology. Frontiers in Pediatrics, 2023; 11: 3389/fped.2023.1226156
- Rynja SP, de Jong TP, Bosch JL, et al. Functional, cosmetic and psychosexual results in adult men who underwent hypospadias correction in childhood. Journal of pediatric urology, 2011; 7(5): 504-515. 1016/j.jpurol.2011.02.008
- van der Horst HJ, de Wall LL. Hypospadias, all there is to know. European Journal of Pediatrics, 2017; 176(4): 435-441. 1007/s00431-017-2864-5
- Hypospadias. https://uroweb.org/guidelines/paediatric-urology/chapter/the-guideline.
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: Opinion. Human Reproduction, 2001; 16(1): 972-978. 1093/humrep/16.5.972
- Xing JS, Bai ZM. Is testicular dysgenesis syndrome a genetic, endocrine, or environmental disease, or an unexplained reproductive disorder? Science direct, 2018; 194: 120-129. 1016/j.lfs.2017.11.039
- Yu X, Nassar N, Mastroiacovo P, et al. Hypospadias Prevalence and Trends in International Birth Defect Surveillance Systems. European Urology, 2019; 76(4): 482-490. 1016/j.eururo.2019.06.027
- Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation, 2012; 84(3): 261-268. 1016/j.diff.2012.06.003
- Castagnetti M, El-Ghoneimi A. Surgical management of primary severe hypospadias in children: Systematic 20-Year review. Journal of Urology, 2012; 184(4): 1469-1474. 1016/j.juro.2010.06.044
- Carmack A, Notini L, Earp BD. Should Surgery for Hypospadias Be Performed Before An Age of Consent? Journal of sex research, 2016; 53(8): 1047-1058. 1080/00224499.2015.1066745
- Rynja SP, de Jong TP, Bosch JL, et al. Functional, cosmetic and psychosexual results in adult men who underwent hypospadias correction in childhood. Journal of Pediatric Urology, 2011; 7(5): 504-515. 1016/j.jpurol.2011.02.008.
- Manzoni G, Bracka A, Palminteri E, et al.: Hypospadias surgery: when, what and by whom? BJU Int, 2004; 94(8): 1188-1195. 1046/j.1464-410x.2004.05128.x
- Thiry S, Saussez T, Dormeus S, et al. Long-Term Functional, Cosmetic and Sexual Outcomes of Hypospadias Correction Performed in Childhood. Urologia Internationalis, 2015; 95(2): 137-141. 1159/000430500

