Kaposi’s Sarcoma: Epidemiological, Clinical and Morphological Studies in the Western and Eastern Parts of the Democratic Republic of Congo
Mulumeoderhwa P1, Chirimwami R1,2,*, Kabamba B3, Van Maanen A4, Fiasse R5 and Marbaix E6
1Pathology Department, Bukavu Catholic University Hospital, Bukavu, DRC
2Pathology Department, Kinshasa University Hospital, Kinshasa, DRC
3Virology Department, St-Luc University Clinics and Catholic University of Louvain, Brussels, Belgium
4Statistical Support Unit, King Albert II Institute of Cancerology and Haematology, St-Luc University Clinics, Brussels, Belgium
5Hepato-gastro-enterology Department, St-Luc University Clinics and Catholic University of Louvain, Brussels, Belgium
6Pathology Department, St-Luc University Clinics and Catholic University of Louvain, Brussels, Belgium
Received Date: 05/06/2024; Published Date: 03/10/2024
*Corresponding author: Raphael Chirimwami, Department of Pathology, Kinshasa University Clinics,
University of Kinshasa (UNIKIN), Mont Amba, Kinshasa XI, P. Box 840, Democratic Republic of the Congo
Abstract
Background: The malignant vascular tumour Kaposi’s sarcoma (KS), previously an endemic disease in the Democratic Republic of the Congo (DRC), is presently an epidemic disease after the advent of Human Immunodeficiency Virus (HIV) in 1981.
Objectives: To compare epidemiological, clinical and histological features of KS in the Western and Eastern regions of the DRC.
Patients and Methods: Retrospective (2000 - 2007) and prospective (2008 - 2015) collection of 194 patients, 65 from Western RDC and 129 from Eastern DRC.
HHV8 immunohistochemical staining allowed us to confirm the diagnosis of KS which was subsequently used for DNA extraction for molecular analysis.
Results: Skin lesions were largely predominant (79.9%). KS was associated with a HIV infection in the vast majority of patients, both in the Western and the Eastern parts of the DRC. However, there was a strong increase in the histological severity of lesions, associated with a striking decrease of the inflammatory infiltrate, in Eastern DRC compared to Western DRC. Uni- and multivariate analyses showed that the main prognostic characteristics were the tumour histological type and the degree of inflammation. Weak inflammatory infiltrate and mixed histological type were both 4 times more likely to occur in the Eastern region while sarcomatous type was 15 times more frequent.
Conclusions: Most KS were associated with HIV immunosuppression in all the DRC, but they are associated with decreased inflammatory response and increased severity of skin lesions in the Eastern part of the country.
Keywords: Kaposi’s sarcoma; Democratic Republic of Congo; Human immunodeficiency virus; Inflammation
Introduction
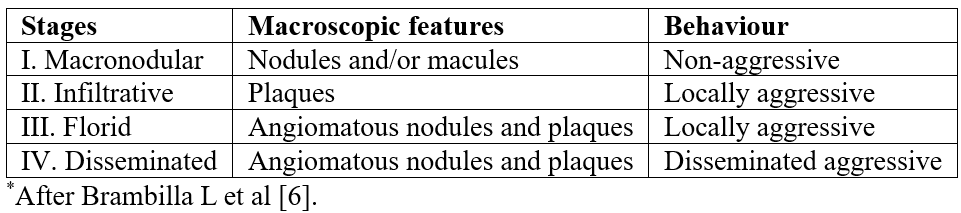
Kaposi’s Sarcoma (KS) is a neoplasm caused by the Human Herpes Virus 8 (HHV-8), of which 4 clinical variants have been described: classic, endemic, epidemic and iatrogenic associated with immunosuppression (Table 1) [1]. While the classic form of KS is restricted to Eastern Europe and Mediterranean countries, its endemic form occurs in Africa and Eastern Europe. However, its epidemic variant, which is the most common malignancy associated with the Acquired Immunodeficiency Syndrome (AIDS) due to the Human Immunodeficiency Virus (HIV) infection worldwide, remains a major scourge in sub-Saharan Africa. Cutaneous lesions are the most frequent and can present as macules, plaques, nodules, lymph-edematous morphological forms, depending on the evolutive stage. They should be distinguished from other entities including pyogenic granuloma, keloid, telangiectasia, hemangioma, and lymphangioma [1-5]. Brambilla et al. proposed a classification where stages I and II combined clinical lesions as macules, patches, warts and isolated or associated nodules while stages III and IV were related to aggressive and often florid muco-cutaneous and lymph-edematous features (Table 2) [6].
Histologically, the lesions are made of spindle-shaped neoplastic cells thought to derive from endothelial cells showing lymphatic differentiation, and vascular slits, often with extravasated erythrocytes, hemosiderin and fibrosis [1,7]. Lesions are categorized as vascular, mixed or sarcomatous histological types according to the predominance of vascular slits or of spindle cells, proliferation of the latter being considered as progression towards a more aggressive type. In addition, many histological variants of KS have been described: anaplastic, lymphangioma-like, lymphangiectatic, bullous, telangiectatic, hyperkeratotic, keloidal, pyogenic granuloma-like, micronodular, ecchymotic, intravascular, and regressing as a result of antiretroviral therapy [4].
Inflammatory cells are almost always present, admixed with neoplastic cells and abnormal vessels in the KS lesion. At the early stage of KS, a rare chronic inflammatory infiltrate can be identified. If the tumour is well developed, it contains a chronic inflammatory infiltrate composed of lymphocytes, plasma cells and dendritic cells. Their density is reduced in AIDS-related KS compared to classic KS lesions, reflecting a decrease in local immune surveillance, allowing a faster growth of KS lesions in HIV patients [8-12].
The absence of epidemiological, clinical, histological and virological studies on KS in the whole DRC, prompted us to compare KS features between the Western and the Eastern regions of this large country.
Table 1: Clinical and epidemiological variants of Kaposi’s sarcoma (KS)*.

Table 2: Pathological stages of KS lesions*.
Patients and Methods
A retrospective study (January 2000 - December 2007) and a prospective study (January 2008 - December 2015) of the clinico-pathological features of KS collected a series of 226 patients in the Western part, referred to Kinshasa, and in the Eastern part, referred to Bukavu in DRC. The ethical committee of the Faculty of Medicine of the Catholic University of Bukavu, DRC, approved these retrospective and prospective studies.
In the retrospective study, 127 cases of KS were retrieved from the 2 pathological laboratories of Kinshasa and Bukavu. However, 18 patients had to be excluded because pathological material was not found in the laboratory archives and 14 patients because medical records did not yield sufficient epidemiological and clinical information, so that 65 patients could be included in the Western region and 30 patients in the Eastern region of DRC. In the prospective study, a questionnaire revealed 99 cases of KS in the Eastern part of DRC.
Overall, the following parameters were recorded: age, sex, location and lesion morphology (size, number, histological type, density of inflammation, hemorrhagic suffusions, mitoses, atypia), duration of disease before the consultation, patient occupation, laboratory tests including the HIV status confirmed by an Elisa HIV rapid test and an UNIGOLD HIV rapid test (both from Trinity Biotech, Wicklow, Eire). The patients with a diagnosis of KS were followed in an outpatient clinic, where previous history and risk factors were sought. Biopsies were taken by the surgeon and/or the dermatologist.
The specimens were fixed in 10% formalin and embedded in paraffin. Histological sections were stained with hematoxylin and eosin (HE). The diagnosis of KS was first done at the Bukavu Catholic University Hospital and thereafter confirmed in the pathology laboratories of the University Hospital of Pavia (Italy) or of the University Hospital St Luc in Bruxelles (Belgium). HHV8 immunohistochemical staining allowed us to confirm the diagnosis of KS which was subsequently used for DNA extraction for molecular analysis.
The abundance of inflammatory cells (macrophages, lymphocytes and polymorphonuclear leukocytes identified according to their histological appearance) was semi-quantitatively estimated on HE-stained sections as weak (less than 10 inflammatory cells per high power field analyzed with x40 objective on an Olympus microscope), moderate (between 10 and 20 inflammatory cells) or abundant (more than 20 inflammatory cells).
Statistical analysis was performed using SAS 9.4 and Epi-info software in its seventh release. Continuous variables were expressed as mean with standard deviation (SD) or medians with range, and the categorical variables as percentages. Statistical comparison for categorical data was done using the Chi-square test (χ2), or a Fisher Exact-test (when number of expected cells <5). Univariate and multivariate logistic regression analyses were performed to identify specific tumour prognostic characteristics. Higher bound for inclusion in the multivariate model was set to 20%. Backward stepwise selection was used to select optimal multivariate model. Results are reported as odds ratio (OR) with 95% confidence intervals (CI). All p values were 2-tailed, and p values of less than 0.05 were considered statistically significant.
Results
In the 2 studies, 194 cases of endemic and epidemic KS could be analyzed during the last 16 years. KS was common, representing 12.3% of the malignant tumours (194/1582) seen over that period. Men were more numerous (n=140 or 72.2%) than women (n=54 or 27.8%) with a sex ratio M/F of 2.6. The mean (± SD) age at the time of consultation was 35.6 (± 14.0) years in men and 29.8 (± 13.2) years in women. Patients were of black race. The province of origin was not specified but the cases were split into 65 patients (33.5%) treated in the Western DRC (Kinshasa) and 129 patients (66.5%) treated in the Eastern DRC (Kivu and other Eastern Provinces). The demography and the KS clinical variants related to the epidemiology of both groups are outlined in Table 3. Although there was no case of endemic KS in the Western DRC while there were 7 cases in the Eastern DRC, the difference was not significant (p=0.098). HHV8 immunohistochemical staining confirmed all the Kaposi’s sarcoma diagnosis.
Tumour characteristics are summarized in Table 4. The vast majority of the lesions were localized in the skin (n=155/194 or 79.9% overall), irrespectively of the region of origin (p=0.069): 61.1% (n=95) in the lower extremities, 16.0% (n=25) in the upper limbs and 22.9% (n=35) in other parts of the body (trunk, head). Extra-cutaneous lesions were mainly localized in lymph nodes (5.7%) and in oral mucosa (2.6%). Skin lesions presented as maculae or erythemato-angiomatous patches, papules, nodules, ulcerative budding lesions and/or chronic lymphedema (Figure 1). The majority of the tumours from the Eastern region were papulo-nodular (74.4%) (Figure 1a, 1b), while 2 types appeared in the West (papulo-nodular 58.9% and ulcerative budding 30.8%) (Figure 1e). Lymphedema was observed in 11.9% of cases (Figure 1e, 1f). The clinical type of the lesion was significantly different between the regions (p=0.013).
Lesions developed with different evolutionary patterns: local aggressiveness (Figure 1c, 1d), dissemination (Figure 1f) and/or spontaneous remission. The changes were slow in the majority of patients (74%). The development was defined by the increase in the number of plaques and/or nodules. The patients had various types of complications related to the aggressiveness of the skin lesions (ulceration, budding, secondary infection, lameness). The duration of the disease after the first consultation was generally long with a median time of 2.5 years (2 months-4 years). Ten patients died of the disease in our series because of respiratory distress in a context of generalized edema, with or without pulmonary embolism, in 7 patients. In 1 patient, the cause of the upper gastro-intestinal bleeding was not investigated. Sixty percent of the patients were ambulant (traders, military servicemen, drivers) and more than half practiced unprotected sex (65.1%) with multiple partners (50.3%). Thirty percent of the patients were agents of customs, teachers, pupils, students or “without occupation”.
Table 4 also compares the stages of clinical severity of the KS skin lesions between patients of the Western and Eastern regions of the DRC, according to Brambilla et al [6]. Advanced KS (stage III) was significantly more frequent in the Eastern region (p<0.0001). In addition, the severity of lesions was increased in patients infected with HIV compared to those not infected in the Eastern region of DRC (p=0.011, Table 5).
In all cases, microscopic analysis (Figure 2, 3) showed the classical histological components: spindle cell population, usually admixed with endothelial cells, hemorrhagic suffusions and inflammatory infiltrate. Mitoses were found in 23.2% of cases (Figure 2c, 3h) and atypias were rare. The histological type was mostly angiomatous in the West while sarcomatous in the East. Semi-quantitative estimation showed that intra-tumoral inflammatory infiltrate was predominantly weak in the East but moderate in the West (p<0.0001, Table 4).
Uni- and multi-variate regression analyses showed that the main prognostic characteristics were the tumour histological type and its inflammatory infiltrate (Table 6). Weak inflammatory infiltrate and the mixed histological type were both 4 times more likely to be found in the Eastern region (p=0.010 and 0.002, respectively) and the odds increase to 15 for the sarcomatous histological type (p<0.001).
Table 3: Demography and epidemiology of 194 patients with KS in the West and East of the DRC.
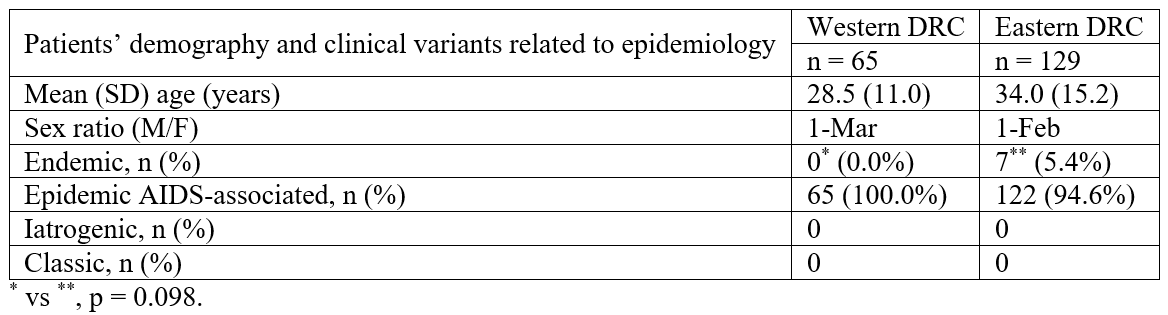
Table 4: Comparison of the tumour characteristics in patients with KS in the Western and the Eastern regions of the DRC.
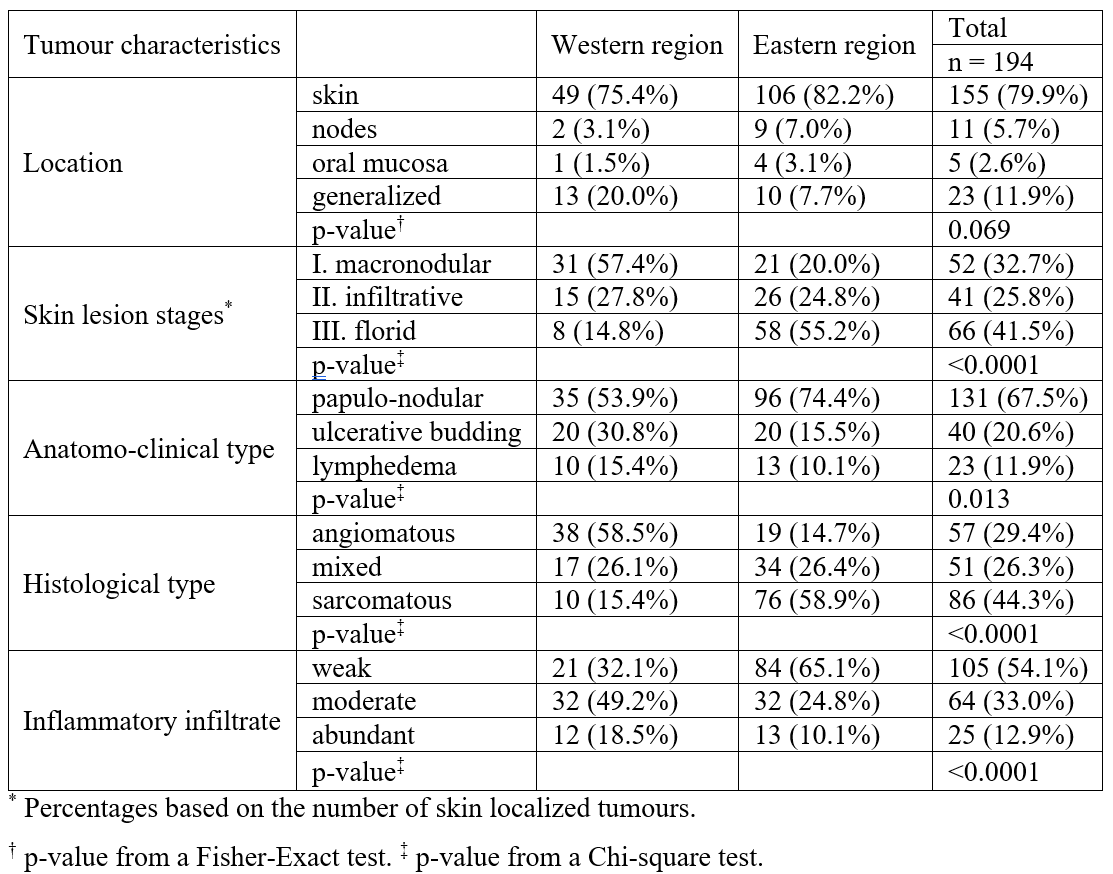
Table 5: Pathological stages of KS related to HIV status in patients in the Eastern region of the DRC.
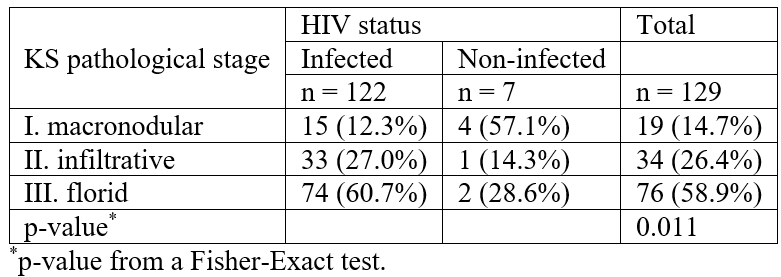
Table 6a: Univariate regression analyses of prognostic characteristics of KS in the Eastern region compared to the Western region.
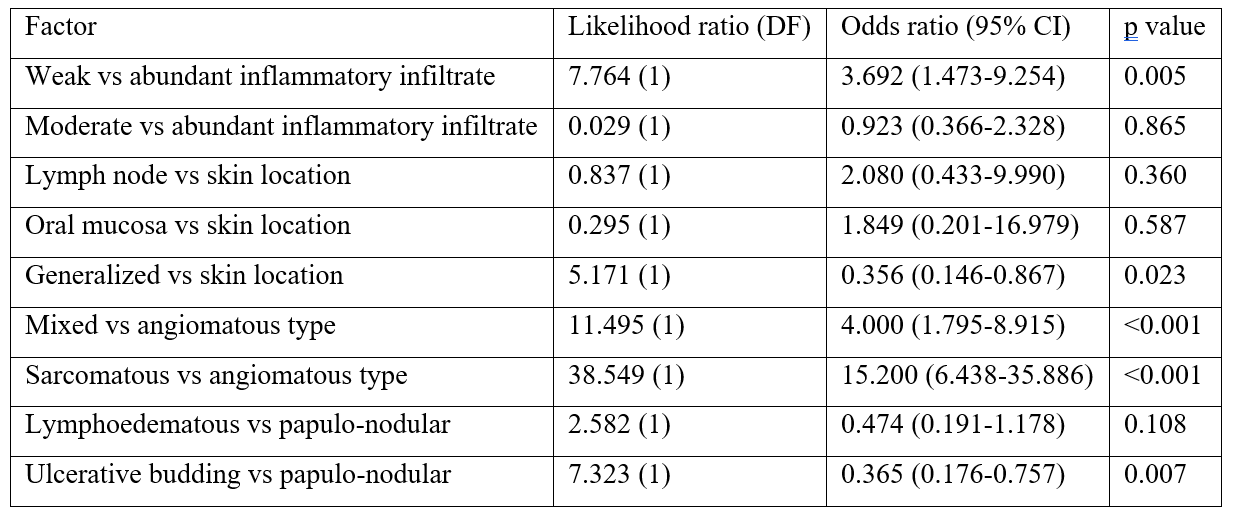
Table 6b: Multivariate regression analysis of prognostic characteristics of KS in the Eastern region compared to the Western region – Best model.
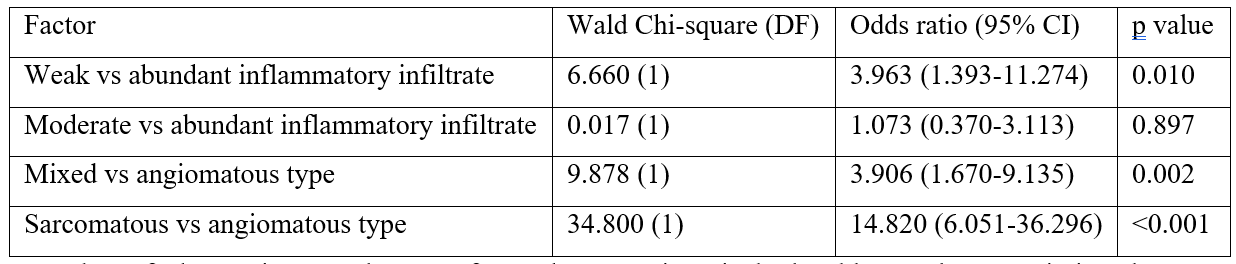
Number of observations used = 194 for each comparison in both tables. Only non-missing data was used. DF=degrees of freedom. CI=confidence intervals.
Higher bound for inclusion in multivariate is set to p=0.20. Best model obtained using a backwards selection process.
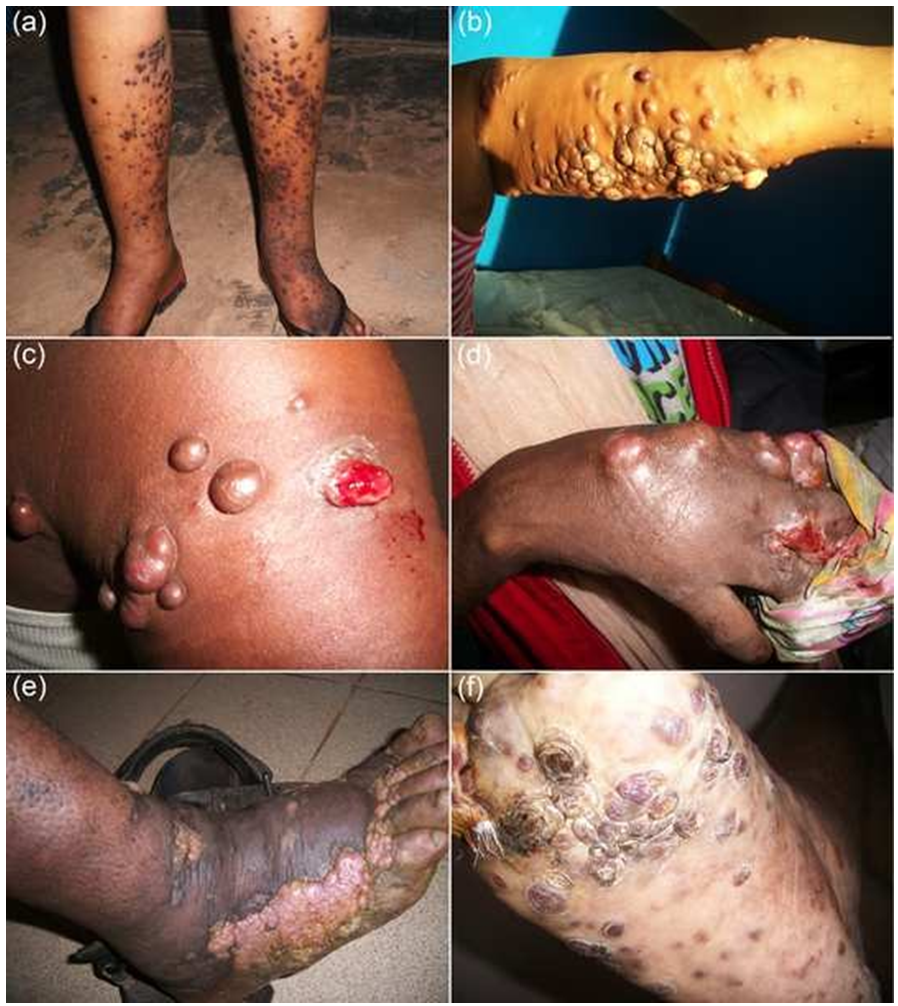
Figures 1: Clinical presentation of some exemplative patients from the Eastern part of the DRC.
(a): Kaposi’s sarcoma (KS) of both legs in a 36-year-old patient without HIV, presenting with papules and nodular pruritic lesions.
(b): KS located in the upper arm of a 30-year-old woman with HIV, after giving birth with breast feeding. KS active lesions for one year.
(c): Multinodular and hemorrhagic lesions located on the shoulder of a 46-year-old patient without HIV.
(d): A 30-year-old patient without HIV.
(e): A 42-year-old patient with HIV, with florid, ulcerative and disseminated lesions in the foot and leg as well as extensive edema.
(f): Multinodular KS disseminated on the leg, ipsilateral and spread to the sole of the foot with lymphadenopathy and lymphedema, in a 54-year-old patient with HIV.
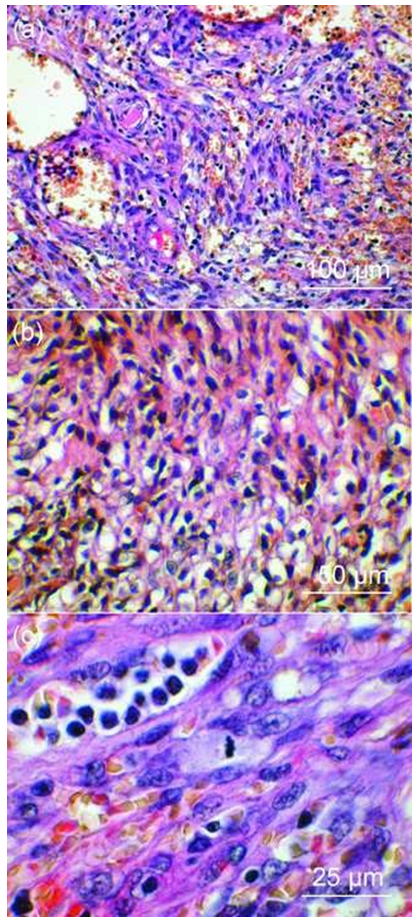
Figures 2: Histological variants of KS (HE staining).
(a): Mixed type of KS in skin papulo-nodular lesions of a 43-year-old man (West DRC), with HIV. Mixed cell population associating diffuse spindle cells, vascular structures and an abundant inflammatory infiltrate.
(b): Sarcomatous KS in skin nodular lesion of a 36-year-old woman (West DRC), with HIV. Spindle cells arranged in swirling bundles or clusters with an abundant inflammatory infiltrate.
(c): Sarcomatous KS in florid and ulcerative skin lesions in a 25-year-old man (East DRC), without HIV. Spindle cells with a mitosis at metaphase, haemorrhagic suffusions and moderate inflammatory infiltrate.
HE staining of all sections.
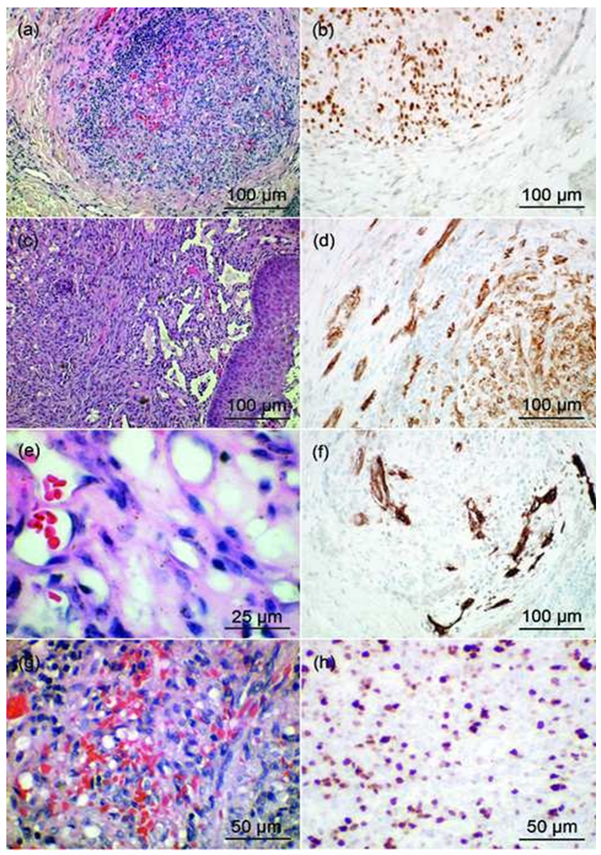
Figures 3: HE staining of KS lesions completed with immunostaining (HHV8, CD31, CD34 and Ki- 67) of patients from various parts of the DRC.
(a) and (c): HE staining of KS sarcomatous type confirmed by HHV8 staining (b)
(e): HE staining of KS angiomatous type and CD31 (d) and CD34 staining (f)
(g): HE staining of KS sarcomatous type and Ki- 67 staining (h)
Discussion
Epidemiology:
Our results show that there was a high frequency of KS cases in the Eastern regions as in the Western regions of the DRC. In all these regions, patients often presented with a late-stage KS and epidemic KS predominated over the endemic KS in the Eastern region, whereas all cases in the Western region were epidemic.
Although there were cases of endemic KS in East Africa, as reported in Uganda [13], the incidence of KS has dramatically increased in Africa, as reported in Zambia since the beginning of the AIDS epidemic in 1983 [14]. Among the 66200 cases of KS reported worldwide, 58800 occurred in sub-Saharan Africa [15,16]. Our study analyzed the epidemiological, clinical and histological aspects of patients with KS associated or not to HIV infection in the DRC and reported a series of severe clinical presentations of this tumor that we met especially in the Eastern part of the DRC.
Clinical and pathological aspects:
Clinically, endemic KS is present mostly in the form of multiple and bilateral skin lesions of the lower limbs as reported by others [17]. Indeed, multiple lesions found in 81.9% of the reported cases were mainly bilateral (70%) and the end of the lower limbs was clearly the most affected location (90%).
The clinical features of epidemic KS vary considerably across the African continent. In a prospective study of unusual clinical cases in northern Nigeria, conducted from September 2003 to August 2005, Kagu et al. found 20 cases of histologically confirmed KS: 17 (85%) men and 3 (15%) women, with a sex ratio 5.7:1, and median age of 37 years (range 21-45 years) [18]. Multiple lesions were a common presentation affecting sites such as the legs, trunk, conjunctiva, upper limbs, rectum, penis, lymph nodes, scrotum and oropharynx.
There were clinical and morphological differences in KS lesions between HIV sero-positive and sero-negative patients in a cohort of 47 patients followed in a hospital in Sydney [19]. The majority of lesions (37/47, 79%) were in late stage, but early stages with predominant vascular clefts were more prevalent in patients with HIV than in those without. The majority of the 33 HIV-infected patients were males less than 60 years old. There were more visceral/extra-cutaneous lesions among these 33 patients than among the patients not infected with HIV. In our series in the East of DRC, 122 patients (94.5%) were HIV sero-positive while only 7 patients (5.4%) were not infected by HIV. The pathological stage of the latter patients was also less aggressive. Conversely, other studies showed that KS lesions in HIV sero-negative subjects were observed in the late stage with high mitotic activity, tumor necrosis and cellular anaplasia compared to KS in HIV sero-positive patients [20].
A retrospective study of Togolese patients with epidemic KS from 2005 to 2011 reported 103 HIV sero-positive patients 36.7 ± 14.9 years-old (mean ± SD), with a sex ratio M/F of 1.1 and 12 sero-negative patients [21]. Lesions were located in the lower limbs (n=76), the mouth mucosa (n=53), the trunk (n=38), and the upper limbs (n=17). There was no significant difference in plasma cells, hyaline globules, extravasation of red blood cells and hemosiderin in lesions between sero-negative and sero-positive patients. Indeed, the four epidemiological forms of KS (classic, endemic, epidemic and iatrogenic) were previously reported to have the same histopathological appearance [1]. Nevertheless, we found more sarcomatous histopathological types in Eastern DRC whereas angiomatous types were more frequent in Western DRC.
Differential diagnosis:
The nodular form of KS usually does not cause diagnostic difficulties. Sometimes, however, a small ulcerated nodular KS may be mistaken for a pyogenic granuloma [3]. The periphery of some nodular lesions of KS may show more dilated vascular spaces suggestive of cavernous hemangioma. These congestive channels are an integral part of the lesion. Major cutaneous nodules are often associated with ulceration. The nodular lesions of KS can be confused histologically with bacillary angiomatosis, other vascular tumours (hemangioma and Kaposiform hemangioma), fibro-histiocytic tumours (e.g., cellular variants and atypical angiomatoid histiocytofibroma), spindle cell melanoma and several other mesenchymal tumours (dermal leiomyosarcoma for instance) [22]. Anaplastic KS, sometimes called polymorphic KS, is poorly documented in the literature, perhaps because of its rarity. This highly malignant form of KS is characterized by an increase in the number of mitoses and marked cellular pleomorphism [4].
Impact of HHV-8 and HIV on immunity and inflammation:
Dendritic cells (DCs) are playing a crucial role in immunity, as antigen-presenting cells able to induce and regulate an adaptive immune response. In a recent review, Campbell et al. hypothesize that DCs have an antiviral function during HHV-8 infection without disease [9]. In case of alteration of immunity such as in a HIV infection, HHV-8 contributes to associated diseases such as lymphoma.
In order to exclude the role of co-infection by HIV, Della Bella et al. investigated DCs in a series of 76 patients with classic KS, thus without HIV infection [12]. They demonstrated that these patients had less myeloid and plasmacytoid DCs than HHV-8 negative healthy controls.
Liu et al. studied the interaction between DCs infected by each virus (HHV-8, HHV-1) for elucidation of pathogenesis in cultures of human peripheral blood mononuclear cells (PBMCs), using flow cytometry [10]. They demonstrated that HHV-8 stimulation could promote the maturation of monocyte-derived DCs and impair their ability to drive the maturation of CD4+ T cells. HHV-8 stimulated monocyte-derived DCs could also capture more HIV-1.
Inflammatory cells are almost always present within KS lesions and depending upon the inflammatory milieu, KS lesions may progress or regress [11]. We found a strikingly decreased inflammatory infiltrate in lesions from Eastern DRC compared to Western DRC, associated with a more aggressive clinical stage and histopathological type, for reasons that remain to be investigated. The abundance of inflammatory cells is linked to altered immunity, particularly by HIV, as observed in this study that showed an association between the pathological severity of KS and the HIV status (p < 0.05).
Therapy:
In the DRC, drugs for chemotherapeutic treatment of KS are not yet available, in contrast to antiretroviral drugs, which may improve the evolution of KS in some patients. It is interesting to mention that the treatment of AIDS-related cutaneous KS can be efficient with a highly active antiretroviral therapy (HAART) associated with microwave therapy of skin lesions, according to Chinese authors [23].
Conclusion
Although we found still a few cases of sporadic KS in the Eastern region of the DRC, the great majority of cases of KS in the Eastern and Western region of the DRC were associated with the epidemic of AIDS.
Most of the patients with KS had skin lesions whose macroscopic severities were related to the HIV status and to the degree of AIDS-related immunosuppression. Lesions in Eastern DRC were in general clinically more advanced, of more aggressive histological type and with lower inflammatory infiltrate than in Western DRC.
The various histological types of Kaposi’s sarcoma diagnosed were confirmed by immunostaining.
Anti-mitotic treatment was not available in the centers of care for patients with KS especially in the Eastern DRC where antiretroviral therapy is the only possible cure presently.
Acknowledgments: The authors wish to thank M. Baguma for the clinical follow-up of patients, F. Cikomola, B. Manwa and M. Paullo for surgical biopsies, S. Wassassia, E. Munguakonkwa for dermatological evaluation of some patients, L. Marot for advises in dermatological pathology and H. Piessevaux for statistical counseling.
Conflicts of interest: None
Funding sources: None
Authors contribution:
- M. designed the study, collected data, analyzed results, wrote drafts of the manuscript and approved the final version of the manuscript
- C. contributed to the design of the study and collection of data, and approved the final version of the manuscript
- K. revised drafts of the manuscript and approved the final version of the manuscript
- V. M. performed statistical analysis of data and approved the final version of the manuscript
- F. contributed to the design of the study, revised drafts of the manuscript and approved the final version of the manuscript
- M. analyzed histological data, revised drafts of the manuscript and approved the final version of the manuscript.
References
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med, 2000; 342: 1027-1038.
- Fatahzadeh M, Schwartz RA. Oral Kaposi's sarcoma: a review and update. Int J Dermatol, 2013; 52: 666-672.
- Scott PL, Motaparthi K, Krishnan B, Hsu S. Pyogenic granuloma-like Kaposi sarcoma: a diagnostic pitfall. Dermatol Online J, 2012; 18: 4.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol, 2008; 3: 31.
- Speicher DJ, Wanzala P, D'Lima M, Njiru A, Chindia M, Dimba E, et al. Diagnostic challenges of oral and cutaneous Kaposi's sarcoma in resource-constrained settings. J Oral Pathol Med, 2015; 44: 842-849.
- Brambilla L, Boneschi V, Taglioni M, Ferrucci S. Staging of classic Kaposi's sarcoma: a useful tool for therapeutic choices. Eur J Dermatol, 2003; 13: 83-86.
- Gutierrez KD, Morris VA, Wu D, Barcy S, Lagunoff M. Ets-1 is required for the activation of VEGFR3 during latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells. J Virol, 2013; 87: 6758-6768.
- Karouni M, Kurban M, Abbas O. Plasmacytoid dendritic cells in skin lesions of classic Kaposi's sarcoma. Arch Dermatol Res, 2016; 308: 487-492.
- Campbell DM, Rappocciolo G, Jenkins FJ, Rinaldo CR. Dendritic cells: key players in human herpesvirus 8 infection and pathogenesis. Front Microbiol, 2014; 5: 452.
- Liu W, Qin Y, Bai L, Lan K, Wang JH. Kaposi's-sarcoma-associated-herpesvirus-activated dendritic cells promote HIV-1 trans-infection and suppress CD4(+) T cell proliferation. Virology, 2013; 440: 150-159.
- Pantanowitz L, Moses AV, Dezube BJ. The inflammatory component of Kaposi sarcoma. Exp Mol Pathol, 2009; 87: 163-165.
- Della Bella S, Nicola S, Brambilla L, Riva A, Ferrucci S, Presicce P, et al. Quantitative and functional defects of dendritic cells in classic Kaposi's sarcoma. Clin Immunol, 2006; 119: 317-329.
- Taylor JF, Templeton AC, Vogel CL, Ziegler JL, Kyalwazi SK. Kaposi's sarcoma in Uganda: a clinico-pathological study. Int J Cancer, 1971; 8: 122-135.
- Bayley AC. Aggressive Kaposi's sarcoma in Zambia, 1983. Lancet, 1984; 1: 1318-1320.
- Mohanlal RD, Pather S. Kaposi's sarcoma, a South African perspective: Demographic and pathological features. S Afr Med J, 2015; 105: 375-378.
- Schulz TF, Cesarman E. Kaposi Sarcoma-associated Herpesvirus: mechanisms of oncogenesis. Curr Opin Virol, 2015; 14: 116-128.
- Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic kaposi sarcoma: epidemiology and risk factors. Cancer, 2000; 88: 500-517.
- Kagu MB, Nggada HA, Garandawa HI, Askira BH, Durosinmi MA. AIDS-associated Kaposi's sarcoma in Northeastern Nigeria. Singapore Med J, 2006; 47: 1069-1074.
- Hong A, Lee CS. Kaposi's sarcoma: clinico-pathological analysis of human immunodeficiency virus (HIV) and non-HIV associated cases. Pathol Oncol Res, 2002; 8: 31-35.
- Santucci M, Pimpinelli N, Moretti S, Giannotti B. Classic and immunodeficiency-associated Kaposi's sarcoma. Clinical, histologic, and immunologic correlations. Arch Pathol Lab Med, 1988; 112: 1214-1220.
- Saka B, Mouhari-Toure A, Wateba IM, Akakpo S, Kombate K, Balaka A, et al. [AIDS related Kaposi sarcoma: 103 cases in dermatology in Lome (Togo)]. Med Sante Trop, 2013; 23: 109-111.
- Forrestel AK, Naujokas A, Martin JN, Maurer TA, McCalmont TH, Laker-Opwonya MO, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care, 2015; 14: 21-25.
- Zheng XK, Lu SH, Liu JF, Lai YR. Clinical and pathological features and treatment of AIDS-related cutaneous Kaposi's sarcoma in Chinese Han patients. Genet Mol Res, 2015; 14: 6830-6837.

