Evaluation of Haemostatic Efficacy and Safety of Surgi-ORC® in Myomectomy Procedures
Diksha Sharma1, Vijay Mate2, Bhavin Trivedi3, Shahid N Karatela4 and Piyush Patel5,*
1Scientist, R&D, Aegis Lifesciences Pvt Ltd, Ahmedabad, India
2Head-RAQA, Aegis Lifesciences Pvt Ltd., Ahmedabad, India
3Manager-RA, Aegis Lifesciences Pvt Ltd., Ahmedabad, India
4Assistant Manager, Aegis Lifesciences Pvt Ltd, Ahmedabad, India
5Head- Quality, Aegis Lifesciences Pvt Ltd., Ahmedabad, India
Received Date: 01/06/2024; Published Date: 03/10/2024
*Corresponding author: Piyush Patel, Head- Quality, Aegis Lifesciences Pvt Ltd, Ahmedabad, India
Abstract
This case study evaluated the efficacy of Surgi-ORC® Original and Surgi-ORC® Non-Woven/SNOW in managing bleeding complications during a myomectomy procedure in a 36-year-old female patient with uterine fibroids. Surgi-ORC® effectively achieved hemostasis within an average time of 1 minute and 6 seconds, without the need for additional interventions. Surgeon-rated handling characteristics were positive, emphasizing ease of application and tissue conformance. Radiological assessments confirmed rapid liquefaction of Surgi-ORC® by Day 2 post-surgery, with no visible traces observed, leading to the presumption of complete absorption and elimination of the need for further radiological examination on Day 28. No complications were observed during the 60-day follow-up, highlighting Surgi-ORC®'s efficacy and safety. The study concluded that these devices offer an effective, safe, and user-friendly solution for controlling bleeding in myomectomy, suggesting their potential as a valuable hemostatic agent in gynecologic surgeries.
Keywords: Haemostat; Myomectomy; Gynecologic surgery; Oxidized regenerated cellulose (ORC); Surgi-ORC® Original; Surgi-ORC® Non-Woven/SNOW; Efficacy
Introduction
Uterine fibroids are prevalent benign gynecologic tumors, affecting an estimated 70% to 80% of women by age 50 [1]. While many women with fibroids may not experience symptoms, approximately one-third of them experience issues such as vaginal bleeding, leading to a need for medical intervention. Myomectomy, a surgical procedure aimed at removing fibroids while preserving the uterus, emerges as a preferred choice for women desiring to maintain fertility [2]. Myomectomy can be performed through various methods, including laparotomy, laparoscopy, or hysteroscopy. However, the procedure comes with challenges, particularly concerning bleeding complications. Conventional laparoscopic cases typically involve around 100 mL of blood loss, while abdominal myomectomy can double that amount as indicated by Barakat et al. [3]. Moreover, a retrospective study highlighted that 6.5% of patients undergoing abdominal myomectomy and 1.1% of those opting for minimally invasive approaches, such as laparoscopic and robot-assisted methods required blood transfusions [3].
To address bleeding complications during myomectomy, various interventions have been developed. These interventions can be categorized into three groups: (1) interventions targeting uterine arteries, such as laparoscopic uterine artery dissection, uterine artery embolization, and hormonal tourniquets like vasopressin and terlipressin. (2) uterotonics like ergometrine, oxytocin, misoprostol, and sulprostone. (3) myoma dissection techniques utilizing tools like lasers and chemical dissectors such as sodium-2-mercaptoethanesulfonate (mesna) [4]. Despite these advancements, excessive hemorrhage remains a significant challenge for gynecologic surgeons performing myomectomies, emphasizing the need for further research and other alternatives in this field.
Topical hemostatic materials, such as oxidized regenerated cellulose (ORC), are increasingly used in various surgeries to control capillary, venous, and small arterial hemorrhages and oozing blood when ligation, electrical coagulation or other conventional methods of control are impractical or ineffective [5]. ORC is a plant-derived hemostatic produced by regenerating pure cellulose into an interwoven fabric, followed by oxidation. It comes in various forms, including Surgi-ORC® Original/Standard (fine weave), Surgi-ORC® Knit (denser weave), Surgi-ORC® Fibril (soft, layered), Surgi-ORC® Non-woven/SNOW (soft, smooth non-woven fabric) and Surgi-ORC® powder as shown in Figure 1, making it easy to handle and apply to irregular surfaces. When left at the surgical site, oxidized cellulose is completely absorbed by the body with minimal tissue reaction [6].
Surgi-ORC® promotes hemostasis by activating the intrinsic coagulation pathway and forming a gelatinous mass upon contact with blood. This mass acts as a matrix for platelet adherence, leading to clot formation. Additionally, the low pH environment from ORC induces vasoconstriction, further reducing bleeding and potentially offering antimicrobial benefits as given in Figure 2 [7,8]. Due to its biocompatibility, bactericidal properties, and ease of use, Surgi-ORC® has gained popularity in surgical practice.
Commercial products based on ORC include Oxycel®, Surgicel®, Evarrest®, ActCel®, Traumacel, Gelita-Cel®, Okcel®, CuraCel®, and Taikeling® [9]. However, intraoperative bleeding remains a concern. Herein, we present a case study evaluating the hemostatic efficacy and handling of Surgi-ORC® Original and Surgi-ORC® Non-Woven/SNOW (Aegis Lifesciences Pvt. Ltd.) during a myomectomy procedure. The study was approved by the Ethics Committee of Shri Shankaracharya Institute of Medical Sciences, Bhilai, India.
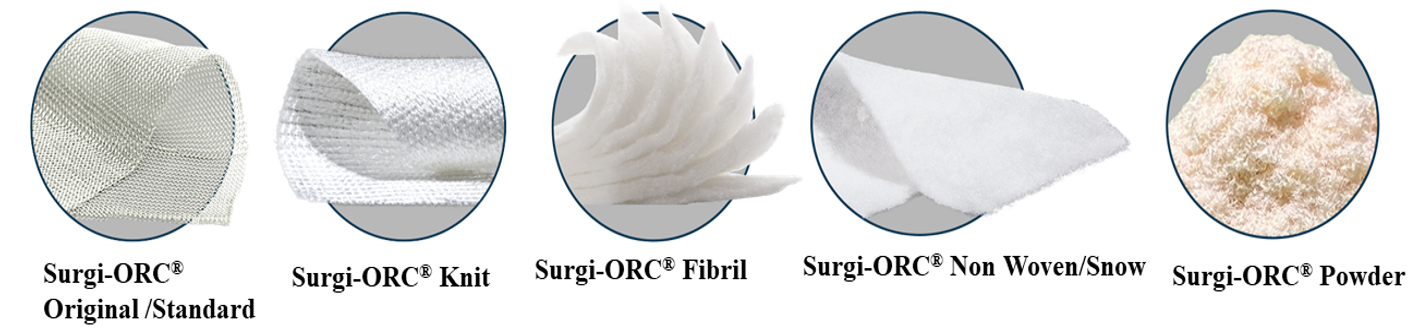
Figure 1: Variants of Surgi-ORC® (Aegis Lifesciences Pvt. Ltd.).
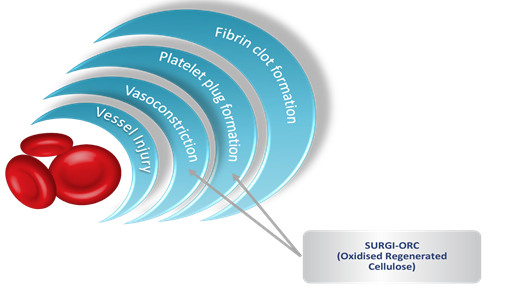
Figure 2: Haemostasis-The Key to Achieving the Right Solution.
Case Presentation
A 36-year-old female patient was admitted to Shri Shankaracharya Institute of Medical Sciences hospital for an open myomectomy surgery aimed at removing uterine fibroids. This clinical case study adhered to ISO 14155:2020, ICH-GCP guidelines, and local regulatory requirements, spanning a duration of 60 days. On the day of the operation, the patient's vital signs, including blood pressure, respiratory rate, temperature, and pulse rate, were evaluated. She had no prior medical history of health complications or comorbidities.
During the procedure, the patient experienced mild bleeding. The surgical wound was located in the abdomen, measuring approximately 3.5 cm with an estimated blood volume loss of 1.5 ml. To control bleeding in the surgical bed, the surgeon utilized two devices: Suri-ORC® Original/Standard and Surgi-ORC® Non-Woven/SNOW (Aegis Lifesciences Pvt. Ltd.), each measuring 1.5 cm × 1.5 cm. Intraoperatively and postoperatively, the patient was prescribed several concomitant medications. Pantop, used for acidity prophylaxis, was administered intravenously at a dosage of 40 mg every 12 hours for three days. Dexa, prescribed for allergic reaction prophylaxis, was administered intravenously at 8 mg every 12 hours. Trenexa, used for bleeding prophylaxis, was administered intravenously at a dosage of 500 mg. Dynapar, a pain relief medication, was administered intramuscularly at a dosage of 75 mg for one day only.
The surgeon evaluated the handling characteristics of Surgi-ORC® during implantation using a questionnaire. The assessment criteria included ease of application to the bleeding site, conformance to tissue surface, ease of delivery to reach the wound site, and ease of preparation for use. Moreover, the assessment of Surgi-ORC® handling was conducted using ratings of excellent, good, or poor. Follow-up evaluations were conducted via radiological examinations on the 2nd, 28th, and 60th days after surgery.
Further, the onset of Surgi-ORC® liquefaction was evaluated through radiological examinations on Day 2 post-procedure, while another assessment was scheduled for Day 28 to confirm complete absorption. Throughout the procedure and up to 60 days post-procedure, the safety of Surgi-ORC® implantation was rigorously monitored to document any complications or adverse reactions. The surgeon proposed that if no traces of the Surgi-ORC® were visible after Day 2, further radiological examination would be unnecessary. Subsequent follow-ups would focus solely on monitoring complications or adverse events during that period.
Discussion
Our study indicates that hemostasis was effectively achieved using the Surgi-ORC® Original and Surgi-ORC® Non-Woven/SNOW devices within an average time of 1 minute and 6 seconds, with hemostasis typically occurring in under 2 minutes. No additional methods, such as electrocoagulation, mechanical means, or pharmacological agents, were needed to control bleeding. According to the surgeon's questionnaire, the hemostatic handling characteristics were rated as good in all aspects, including application, conformance to tissue surface, delivery to the wound site, and preparation for use. There was no observed post-operative drainage.
On Day 2, it was observed that liquefication of Surgi-ORC® started and ORC was no longer visible post-surgery when examined under X-ray as depicted in Figure 3. The wound showed signs of healing progress. There was no need for further surgery due to re-bleeding or additional dressing reinforcement. The patient did not experience any adverse event or complication during the post-operative period. Owing to the absence of ORC traces on Day 2, no radiological examination was conducted on Day 28, with the surgeon presuming complete absorption of the ORC product by that time. Further, on day 60, no adverse events or complications were observed.
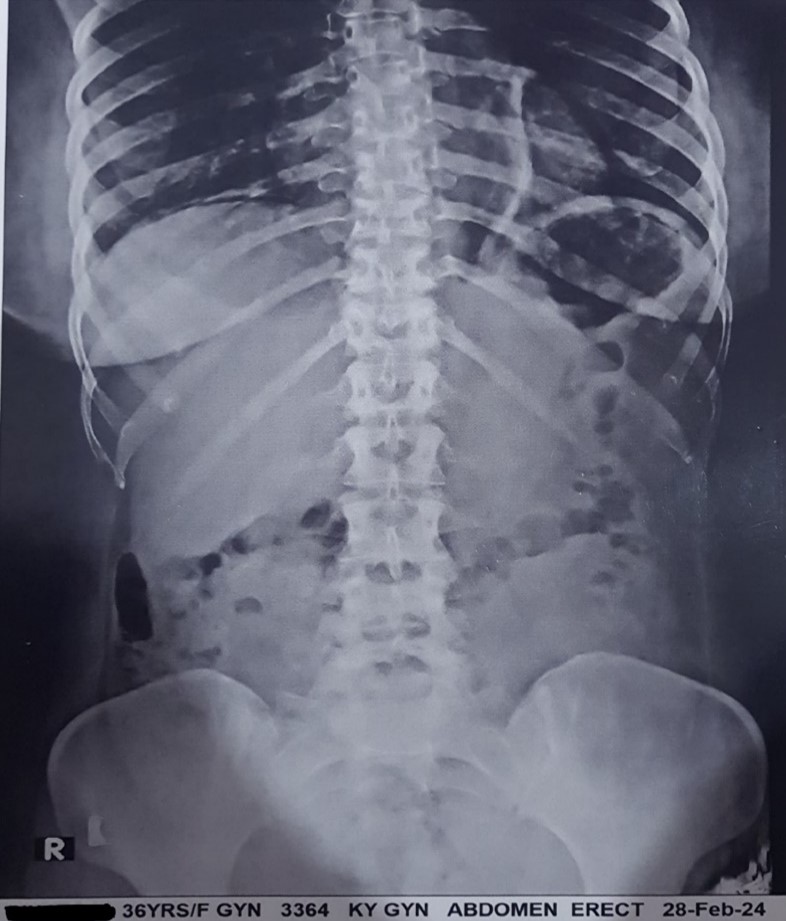
Figure 3: Post-surgery X-ray shows no visible traces of Surgi-ORC® on Day 2.
Conclusion
In conclusion, the utilization of Surgi-ORC® Original and Surgi-ORC® Non-Woven/SNOW devices resulted in effective hemostasis during the open myomectomy surgery, with an average time of 1 minute and 6 seconds for achieving haemostasis. The handling characteristics of Surgi-ORC® were consistently rated as good, highlighting its ease of application and adaptability. Radiological assessments confirmed the rapid liquefaction of Surgi-ORC® by Day 2 post-surgery, with no visible traces of the haemostat observed. This led the surgeon to presume complete absorption, eliminating the necessity for further radiological examination on Day 28. Furthermore, no complications were observed throughout the 60-day postoperative period. Their ease of use, proven biocompatibility, and safety profile make them a valuable addition to the surgical toolbox for gynaecologic surgeons. Further studies with larger sample sizes and longer follow-up periods may provide additional insights into their long-term efficacy and safety.
Conflict of interest: The authors have no conflict of interests.
References
- Hickman LC, Kotlyar A, Shue S, Falcone T. Hemostatic techniques for myomectomy: an evidence-based approach. Journal of minimally invasive gynecology, 2016; 23(4): 497-504.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane database of systematic reviews, 2014; 8.
- Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: A comparison of surgical outcomes. Obstetrics & Gynecology, 2011; 117(2 Part 1): 256-266.
- Raga F, Sanz-Cortes M, Bonilla F, Casañ EM, Bonilla-Musoles F. Reducing blood loss at myomectomy with use of a gelatin-thrombin matrix hemostatic sealant. Fertility and sterility, 2009; 92(1): 356-360.
- Wang H, Chen P. Surgicel® (oxidized regenerated cellulose) granuloma mimicking local recurrent gastrointestinal stromal tumor: A case report. Oncology letters, 2013; 5(5): 1497-1500.
- Frati A, Thomassin-Naggara I, Bazot M, Daraï E, Rouzier R, Chéreau E. Accuracy of diagnosis on CT scan of Surgicel® Fibrillar: results of a prospective blind reading study. European Journal of Obstetrics & Gynecology and Reproductive Biology, 2013; 169(2): 397-401.
- Tam T, Harkins G, Dykes T, Gockley A, Davies M. Oxidized regenerated cellulose resembling vaginal cuff abscess. JSLS: Journal of the Society of Laparoendoscopic Surgeons, 2014; 18(2): 353.
- Qian Z, Xiong F, Xia X, Gu P, Wang Q, Wu A, et al. Clinical and economic impact of oxidized regenerated cellulose for surgeries in a Chinese tertiary care hospital. Journal of Comparative Effectiveness Research, 2020; 9(15): 1079-1090.
- Rodić-Grabovac B, Sailović P, Lipić N. Medical and pharmaceutical application of oxidized cellulose. Journal of Chemists, Technologists and Environmentalists, 2020; 1(1): 1-11.

