A Case Report on Systemic Lupus Erythematosus-Associated Antiphospholipid Syndrome and Hyperhomocysteinemia
Sophia Echevarria1, Eric Johnson2, Omadevi Dhanraj2, Kathryn Provoast3, Sakeena H Rizvi4, Rafiq J Baksh5, Zafar Qureshi6,* and Syed A A Rizvi7,*
1Universidad Mayor de San Simón, Cochabamba, Bolivia
2Ross University School of Medicine, Miramar, Florida, USA
3Barry University, Miami, Florida, USA
4Adlai E. Stevenson High School, Lincolnshire, IL, USA
5Maimonides Midwood Community Hospital, Brooklyn, New York, USA
6UMC Free Clinic, Miami Gardens, Florida, USA
7College of Biomedical Sciences, Larkin University, Miami, Florida, USA
Received Date: 17/05/2024; Published Date: 01/10/2024
*Corresponding author: Syed A A Rizvi, MD, PhD, MPH, MBA, College of Biomedical Sciences, Larkin University, 18301 N Miami Ave, Miami, FL 33169, USA
&
Zafar Qureshi, MD, UMC Free Clinic, 700 NW 183rd St, Suite B, Miami Gardens, Florida, USA
Abstract
Systemic Lupus Erythematosus (SLE) associated with antiphospholipid syndrome and hyperhomocysteinemia are all complex and potentially life-threatening conditions that affect the immune system and cardiovascular health. SLE is an autoimmune disorder in which the body's immune system mistakenly attacks healthy tissue, leading to inflammation and damage in various organs and tissues. Antiphospholipid syndrome is when the immune system produces abnormal antibodies that attack phospholipids, a type of fat in the blood that plays a key role in blood clotting. This can lead to an increased risk of blood clots, stroke, and other complications. Hyperhomocysteinemia is a genetic disorder in which the body cannot properly break down the amino acid homocysteine or from vitamin deficiencies, leading to elevated homocysteine levels, and a buildup of this toxic substance in the blood and tissues. The three conditions can often coexist in individuals, creating a complex and challenging clinical picture for healthcare providers. People with lupus erythematosus are at an increased risk of developing antiphospholipid syndrome, as the autoimmune process underlying lupus can trigger the production of abnormal antibodies. Additionally, both lupus and antiphospholipid syndrome can lead to vascular damage and inflammation, increasing the risk of developing hyperhomocysteinemia or homocystinuria. Managing these conditions requires a multidisciplinary approach that includes close monitoring of symptoms, regular blood tests to assess disease activity, and a personalized treatment plan that may include medications to suppress the immune system, anticoagulants to prevent blood clots, and dietary modifications to reduce homocysteine levels. Overall, the coexistence of lupus erythematosus, antiphospholipid syndrome, and homocystinuria presents a complex and challenging clinical scenario that requires specialized care and expertise. Through ongoing research and collaboration among healthcare providers, scientists, and patients, we can continue to improve our understanding of these conditions and develop more effective treatments to improve outcomes and quality of life for individuals affected by these disorders. It is essential for healthcare professionals to stay current on the latest research and guidelines to provide the best possible care for patients with these complex autoimmune and genetic conditions.
Introduction
Systemic Lupus Erythematosus is a multisystem autoimmune disease of variable clinical presentation and unknown etiology. SLE disproportionately affects women (10:1); specifically highest in women of African descent [1]. SLE is diagnosed based on clinical findings and laboratory evidence such as antiphospholipid antibodies, complement protein levels, and SLE-specific antibodies (e.g., anti-dsDNA antibodies, Anti-Sm antibodies) [2]. SLE is most commonly complicated by cardiovascular disease, infection, and renal disease. A potential contributor to the increased risk of cardiovascular complication is elevated serum homocysteine and low vitamin B12 levels found in patients with SLE compared to individuals without SLE [3].
Hyperhomocysteinemia is defined as a concentration greater than 15 nmol/mL of homocysteine in the blood and can be classified as moderate (15-30 nmol/mL), intermediate (30-100 nmol/mL), and severe (>100 nmol/mL) [4,5]. Homocysteine is a metabolite needed for the endogenous synthesis of methionine and plays a vital role in the folate cycle. Homocysteine can be recycled into methionine or degraded into cysteine depending on the availability of vitamins B6 (pyridoxine), B9 (folate), and B12 (Cobalamin) [6]. Inadequate enzyme activity due to genetic defects and vitamin deficiencies can lead to elevated homocysteine levels. Additional causes correlated with elevated homocysteine are chronic renal failure, hypothyroidism, cigarette smoking, malignant tumors, and certain medications [6].
Elevated levels of homocysteine increase the risk of cardiovascular, cerebrovascular, and thromboembolic disease [7]. Hyperhomocysteinemia is currently treated with vitamin supplementation; however, further research is needed to investigate the efficacy of vitamin therapy in diminishing the risk of cardiovascular complications in patients with hyperhomocysteinemia [8].
Case Presentation
A 53-year-old female with no significant past medical history presented to the clinic on March 30th, 2022, due to a complaint of right eye redness and pain for the past week. The patient denied any history of injury. The patient also reported two episodes of dizziness leading to nausea and vomiting. During the visit, the patient’s blood pressure was elevated (160/104 mmHg). The patient was given polytrim eye drops and confirmed she was not taking any medication. On April 6, the patient visited the clinic for a follow-up of the eye redness, which had improved, and was subsequently started on Lisinopril-Hydrochlorothiazide 10-12.5 mg due to continuous high blood pressure. Routine labs (CBC, CMP, UA, TSH, Free T4, Lipid panel, and HBA1c) were ordered during the visit.
One week later, on April 13th, 2022, the patient's laboratory results were reviewed, revealing the presence of a urinary tract infection in her urine analysis with positive leukocyte esterase and red blood cells. The patient denied dysuria, increased frequency/urgency or gross hematuria. She was started on sulfamethoxazole- trimethoprim (Bactrim) twice a day for 10 days. During her subsequent appointment, the patient reported experiencing vomiting and diarrhea on the fourth of her prescribed 10-day course of Bactrim. She subsequently stopped the medication on the sixth day. The patient also expressed feelings of fatigue, a sensation of heaviness in her head and daily vomiting and abdominal cramping. Therefore, the decision was made to discontinue the use of Bactrim.
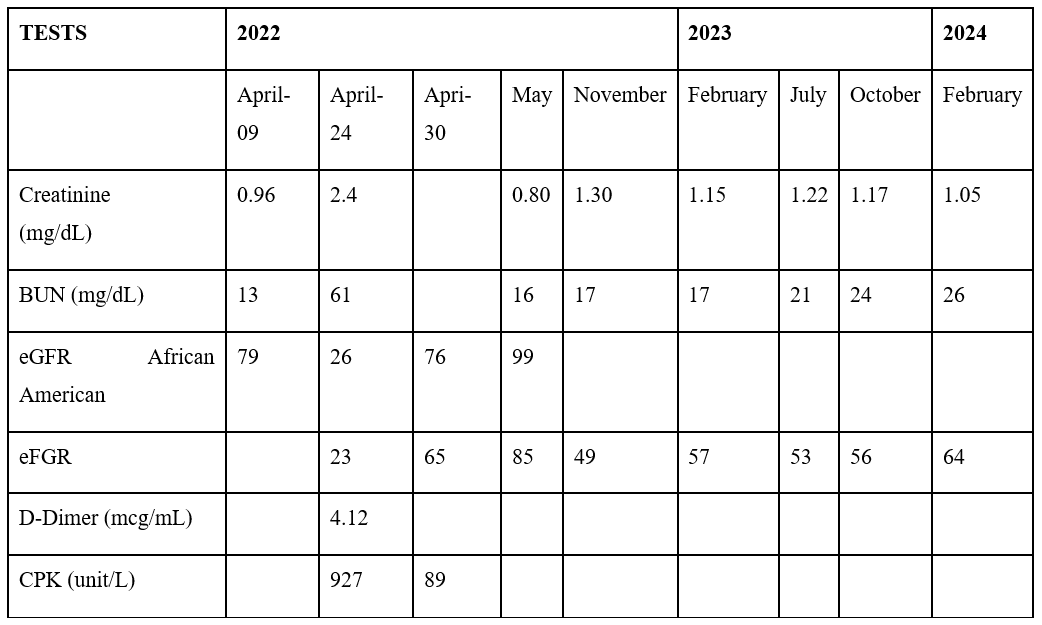
The patient presented to the emergency department on April 24th, 2022, complaining of worsening confusion and loss of appetite. Convulsion was witnessed while in the nursing unit. Initial labs were significant for elevated D-dimer, elevated creatine phosphokinase, acute kidney injury, elevated homocysteine, leukopenia and thrombocytopenia. Chest x-ray and axial CT without contrast images were obtained with no acute findings or abnormalities. She was admitted for metabolic encephalopathy and seizure disorder. Additional testing revealed positive antinuclear antibodies (ANA), anti-Sjogren’s syndrome A (Anti-SSA), and lupus anticoagulant antibodies. MRI of the brain showed white matter disease compatible with multiple sclerosis as other demyelinating processes from immune, viral, or toxic etiology as well as acute disseminated encephalomyelitis (Table 1).
Table 1: Autoimmune panel.
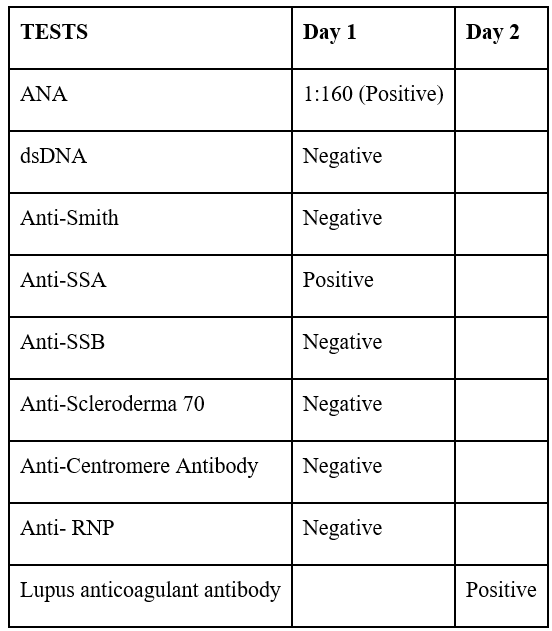
On May 7th, 2022, the patient returned for a follow-up appointment after being hospitalized from April 23rd, 2022 - May 2nd, 2022. She was recommended to maintain regular follow-ups with her primary care physician as needed. Furthermore, it was recommended for the patient to pursue additional evaluation and management by scheduling follow-up appointments with her neurologist and rheumatologist. Upon her return, the rheumatologist prescribed her prednisone and hydroxychloroquine, while the neurologist indicated vitamin B1 and B6 supplementation alongside her existing hypertension and statin medications. On November 11, 2023, the neurologist recommended magnetic resonance angiography (MRA) per patient. Subsequently, on December 23, 2023, the diagnosis of lacunar infarction and stenosis of the precerebral artery was confirmed. Despite persistently elevated homocystinuria levels (18-14 mcmol/L) gradually decreasing, the patient reports improved well-being without any concurrent complaints (Tables 2, 3).
Table 2: Inflammatory panel.
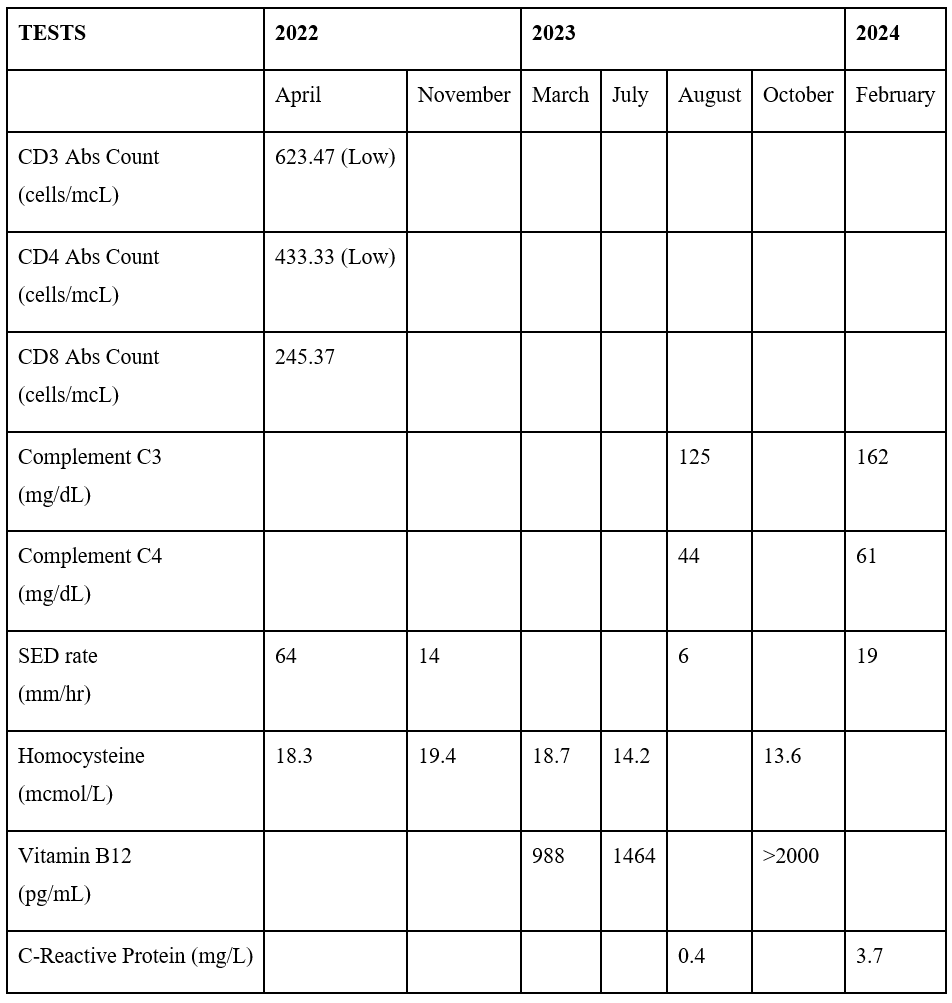
Table3: General Chemistry Panel.
Discussion
Hyperhomocysteinemia is present in approximately 15% of patients with systemic lupus erythematosus (SLE) [9]. One proposed mechanism of elevated homocysteine is a deficiency of folate, B6, and B12, vitamins noted to be essential to homocysteine metabolism [11]. Another route for pathogenesis stems from diminished renal function, such as chronic kidney disease (CKD) [12]. Approximately 70% of accumulated homocysteine is eliminated through the kidneys each day, primarily via the transsulfuration (TS) pathway [13, 14], and patients with CKD have demonstrated impaired metabolic clearance of homocysteine by both TS and remethylation (RM) pathways [15]. Lastly, severe levels of hyperhomocysteinemia are seen in patients with a genetic mutation of the enzyme cystathionine β-synthase or methylenetetrahydrofolate reductase [10], which further implicates processes of TS, RM, and transmethylation in the roll of pathogenesis. Although there is still debate about the exact mechanism by which SLE commonly produces elevated homocysteine levels, one of the aforementioned pathways is likely involved.
SLE stands as the disease most commonly associated with Antiphospholipid Syndrome (APS), present in approximately 35 percent of cases [33]. APS manifests clinically with recurring venous, arterial, and/or small vessel thrombosis (e.g., DVTs, stroke, TIAs), along with specific pregnancy complications such as recurrent miscarriages, and premature births. A clinical diagnosis of APS is supported by the presence of at least one or more types of antiphospholipid antibody (e.g., lupus anticoagulant, anticardiolipin antibodies, Anti-β2-glycoprotein antibodies) in symptomatic patients [34,35]. The management of acute thrombotic events (e.g., stroke) varies depending on the individual patient’s clinical presentation. In contrast, long-term management of APS concentrates on initiating thromboprophylaxis through systemic antiplatelet and anticoagulation therapy, such as low-dose aspirin, warfarin, or heparin therapy [35,36].
Prompt and individualized treatment is required in SLE patients to treat the variable nature of the disease properly. SLE affects an array of organs, including the kidneys, musculoskeletal system, central nervous system, and cardiovascular system. The 10-year survival rate for patients with SLE has gradually increased over time and is currently at about 90%, suggesting that the management of the condition has improved [18,19]. Mortality in patients with SLE typically occurs due to cardiovascular complications, neoplasm, active SLE, and infectious disease [16,18]. The goals in the treatment of SLE are to promote prolonged survival, safeguard against organ impairment, and enhance overall quality of life. This objective is achieved by effectively managing disease activity, reducing the occurrence of comorbidities, and mitigating adverse effects associated with medications. Regular monitoring of the disease process and its response to medication is required [17].
Treatment regimens will depend on the severity of the disease status. Several indices have been developed to aid with the assessment of SLE status in patients. The SEDAI-2K, BILAG, and SLAM-R are SLE scales useful for the assessment of global disease, systemic involvement, and disease severity, respectively, and have demonstrated validity and reliability [20]. A hallmark of the management of SLE especially for patients currently asymptomatic, in disease remission, or with mild symptoms is to focus on preventative care, which includes the avoidance of triggers for flare-ups as well as screening for conditions commonly associated with SLE. Patients should seek to mitigate triggers such as UV light exposure and smoking [21,22]. Likewise, patients should be sure to acquire necessary immunizations and screen for complications of SLE such as CVD, osteoporosis, and malignancy [23] while also maintaining a healthy diet and exercising regularly.
Regardless of disease status, there is strong evidence supporting the pharmacological use of antimalarials, especially hydroxychloroquine, as a first-line option for the prevention and treatment of disease [24]. Further treatment is tailored to the individual's presenting symptoms, organ involvement, and severity and may necessitate corticosteroids, NSAIDS, and antiproliferative immunosuppressants. Stepwise increases in pharmacological treatment should be provided as disease severity intensifies while using the lowest necessary dose of corticosteroids to minimize medication toxicity. This judicious use of corticosteroids is to prevent the likelihood of osteoporosis and CVD. The starting suggested dosage of corticosteroids in patients with mild cases is 7.5mg daily and then titrated as needed to control the disease. When hydroxychloroquine and corticosteroids are insufficient, antiproliferative immunosuppressants such as azathioprine, methotrexate, and mycophenolate should be used [33]. Special consideration is needed to treat specific organ involvement and symptoms due to the variable nature of disease manifestation.
Monitoring of SLE after diagnosis should take place every 3 to 6 months depending on severity and even more frequently in patients with lupus nephritis. Regular monitoring should include a review of medical history, physical exam, and screening for comorbidities related to disease and medication regimen. Laboratory values useful in disease monitoring include CBC, acute phase reactants ESR and CRP, UA with sediment, creatinine, protein to creatinine ratio, dsDNA, and C3 and C4 complement levels [26]. Hyperhomocysteinemia has been documented to be associated with several adverse conditions, especially vascular complications such as coronary heart disease and stroke [27,28]. Normal homocysteine concentrations range between 5 and 15 micromol/L whereas hyperhomocysteinemia is classified as moderate from 15 to 30 micromol/L, intermediate from 30 to 100 micromol/L, and severe >100 micromol/L [32]. Folate, cobalamin, and betaine have been shown to correct elevated levels of homocysteine [29]. However, it has not been shown that correction of homocysteine levels improves a patient’s risk for coronary artery disease, peripheral artery disease, or thrombus formation and is only minimally beneficial in stroke prevention [30,31].
Based on the patient’s records, the patient’s status should be considered mild and stable. The acute kidney injury has resolved, the WBC and platelets are trending positive toward baseline, and the patient’s mental status has returned to baseline. In the future, the patient must follow up regularly with a rheumatologist and any additional healthcare provider as required by disease presentation. Periodic immunological measurements must be taken to monitor the patient’s disease condition, along with clinical evaluation and interviews detailing disease status. Additionally, the patient should adhere to the medication regimen prescribed by their team of healthcare providers which will likely be adjusted as needed for adequate control of their condition and to ensure medication tolerance.
Conclusion
In conclusion, after hospital discharge, the patient-maintained follow-up appointments with rheumatology and neurology specialists. These practitioners diligently continued to monitor her condition and adjusted her medication regimen as needed. Although her homocystinuria levels remained elevated, a downward trend was observed, suggesting a positive response to treatment. Currently, the patient’s prognosis is favorable, and her clinical status remains stable.
References
- Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Seminars in Arthritis and Rheumatism, 2010; 39(4): 257-268. doi: 10.1016/j.semarthrit.2008.10.007.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Annals of the Rheumatic Diseases, 2019; 78(6): 736-745. doi: 10.1136/annrheumdis-2019-215089.
- Tsai T-Y, Lee T-H, Wang H-H, Yang T-H, Chang I-J, Huang Y-C. Serum homocysteine, folate, and vitamin B12 levels in patients with systemic lupus erythematosus: A meta-analysis and meta-regression. Journal of the American College of Nutrition, 2020; 40(5): 443-453. doi: 10.1080/07315724.2020.1788472.
- Son P, Lewis L. Hyperhomocysteinemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024.
- Morris AA, Kožich V, Santra S, et al. Guidelines for the diagnosis and management of Cystathionine Beta‐synthase deficiency. Journal of Inherited Metabolic Disease, 2016; 40(1): 49-74. doi: 10.1007/s10545-016-9979-0.
- Kim J, Kim H, Roh H, Kwon Y. Causes of hyperhomocysteinemia and its pathological significance. Archives of Pharmacal Research, 2018; 41(4): 372-383. doi: 10.1007/s12272-018-1016-4.
- Poddar R. Hyperhomocysteinemia is an emerging comorbidity in ischemic stroke. Experimental Neurology, 2021; 336: 113541. doi: 10.1016/j.expneurol.2020.113541.
- Guéant J-L, Guéant-Rodriguez R-M, Oussalah A, Zuily S, Rosenberg I. Hyperhomocysteinemia in cardiovascular diseases: Revisiting observational studies and clinical trials. Thrombosis and Haemostasis, 2022; 123(03): 270-282. doi: 10.1055/a-1952-1946.
- Timlin H, Manno R, Douglas H. Hyperhomocysteinemia and Lupus Nephritis. Cureus, 2019; 11(7): e5065. doi: 10.7759/cureus.5065.
- Leclerc D, Sibani S, Rozen R. Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR) and Overview of Mutations/Polymorphisms. In: Madame Curie Bioscience Database. Austin (TX): Landes Bioscience; 2000-2013.
- Robertson J, Iemolo F, Stabler SP, Allen RH, Spence JD. Vitamin B12, homocysteine and carotid plaque in the era of folic acid fortification of enriched cereal grain products. CMAJ, 2005; 172(12): 1569-1573. doi: 10.1503/cmaj.045055.
- van Guldener C, Robinson K. Homocysteine and renal disease. Semin Thromb Hemost, 2000; 26(3): 313-324. doi: 10.1055/s-2000-8407.
- Durand P, Prost M, Loreau N, Blache D. Impaired Homocysteine Metabolism and Atherothrombotic Disease. Laboratory Investigation, 2001; 81(5): 645-672. https://doi.org/10.1038/labinvest.3780275.
- Li N, Chen L, Muh RW, Li PL. Hyperhomocysteinemia associated with decreased renal transsulfuration activity in Dahl S rats. Hypertension, 2006; 47(6): 1094-1100. doi: 10.1161/01.HYP.0000219634.83928.6e.
- Stam F, van Guldener C, ter Wee PM, et al. Homocysteine clearance and methylation flux rates in health and end-stage renal disease: association with S-adenosylhomocysteine. Am J Physiol Renal Physiol, 2004; 287(2): F215-F223. doi: 10.1152/ajprenal.00376.2003.
- Zen M, Salmaso L, Barbiellini Amidei C, et al. Mortality and causes of death in systemic lupus erythematosus over the last decade: Data from a large population-based study. Eur J Intern Med, 2023; 112: 45-51. doi: 10.1016/j.ejim.2023.02.004.
- van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis, 2014; 73(6): 958-967. doi:10.1136/annrheumdis-2013-205139.
- Cervera Ricard, Khamashta Munther A, Font Josep, Sebastiani Gian Domenico, Gil Antonio, Lavilla Paz, et al. European Working Party on Systemic Lupus Erythematosus. Morbidity and Mortality in Systemic Lupus Erythematosus During a 10-Year Period: A Comparison of Early and Late Manifestations in a Cohort of 1,000 Patients. Medicine, 2003; 82(5): p 299-308. DOI: 10.1097/01.md.0000091181.93122.55.
- Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore), 2006; 85(3): 147-156. doi: 10.1097/01.md.0000224709.70133.f7.
- Romero-Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus: updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Arthritis Care Res (Hoboken), 2011; 63 Suppl 11(0 11): S37-S46. doi: 10.1002/acr.20572.
- Lehmann P, Homey B. Clinic and pathophysiology of photosensitivity in lupus erythematosus. Autoimmun Rev, 2009; 8(6): 456-461. doi: 10.1016/j.autrev.2008.12.012.
- Ghaussy NO, Sibbitt W Jr, Bankhurst AD, Qualls CR. Cigarette smoking and disease activity in systemic lupus erythematosus. J Rheumatol, 2003; 30(6): 1215-1221.
- Chevet B, Figueroa-Parra G, Yang JX, et al. Utilization of preventive services in a systemic lupus erythematosus population-based cohort: a Lupus Midwest Network (LUMEN) study. Arthritis Res Ther, 2022; 24: 211. https://doi.org/10.1186/s13075-022-02878-8.
- Sisó A, Ramos-Casals M, Bové A, et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus, 2008; 17(4): 281-288. doi: 10.1177/0961203307086503.
- McKeon KP, Jiang SH. Treatment of systemic lupus erythematosus. Aust Prescr, 2020; 43(3): 85-90. doi: 10.18773/austprescr.2020.022
- Kuhn A, Bonsmann G, Anders HJ, Herzer P, Tenbrock K, Schneider M. The Diagnosis and Treatment of Systemic Lupus Erythematosus. Dtsch Arztebl Int, 2015; 112(25): 423-432. doi: 10.3238/arztebl.2015.0423.
- Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc, 2008; 83(11): 1203-1212. doi: 10.4065/83.11.1203.
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA, 2002; 288(16): 2015-2022. doi: 10.1001/jama.288.16.2015.
- Kang SS. Treatment of hyperhomocyst(e)inemia: physiological basis. J Nutr, 1996; 126(4 Suppl): 1273S-5S. doi: 10.1093/jn/126.suppl_4.1273S.
- Ray JG, Kearon C, Yi Q, Sheridan P, Lonn E. Heart Outcomes Prevention Evaluation 2 (HOPE-2) Investigators. Homocysteine-lowering therapy and risk for venous thromboembolism: a randomized trial. Ann Intern Med, 2007; 146(11): 761-767. doi: 10.7326/0003-4819-146-11-200706050-00157.
- Martí-Carvajal AJ, Solà I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev, 2017; 8(8): CD006612. doi: 10.1002/14651858.CD006612.pub5.
- Kang SS, Wong PW, Malinow MR. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr, 1992; 12: 279-298. doi: 10.1146/annurev.nu.12.070192.001431.
- Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis, 2015; 74: 1011.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost, 2006; 4(2): p.295-306. doi: 1111/j.1538-7836.2006.01753.x.
- Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med, 2018; 378(21): p.2010-2021. doi: 1056/nejmra1705454.
- Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis, 2019; 78(10): p.1296-1304. doi: 1136/annrheumdis-2019-215213.

