A Remarkable Case Report of Splenic Lymphoma with Villous Lymphocytes in a Male Sudanese Patient
Bashir Abdrhman Bashir*
Department of Hematology, Faculty of Medical Laboratory Sciences, Port Sudan Ahlia University
Received Date: 27/04/2024; Published Date: 23/09/2024
*Corresponding author: Dr. Bashir Abdrhman Bashir, Associate Professor of Hematology, Chairman of Hematology Department, Faculty of Medical Laboratory Sciences, Port Sudan Ahlia University, Port Sudan, Sudan
Abstract
Background: Splenic lymphoma with villous lymphocytes (SLVL) is an unusual lymphoid tumor that accounts for <1% of all lymphoproliferative neoplasms. It is the leukemic analog of splenic marginal zone lymphoma (SMZL) and is distinguished by splenomegaly, frequently with no lymphadenopathy, significant lymphocytosis, and villous lymphocytes on microscopic examination. This condition is often mistaken with other chronic lymphoproliferative illnesses and must be distinguished from them.
Case presentation: Here, we present a case of a 67-year-old man who had no lymphadenopathy but had a huge splenomegaly [18 cm] below the costal edge on inspection. The WBC count was 87.0x109/L, with an absolute lymphocyte count of 72.6x109/L. Peripheral smear examination revealed that 83% of the cells were lymphoid, with 55% having a villous appearance. These villous lymphocytes were also seen in bone marrow aspirate and biopsy. All cytochemical stains, even tartrate-resistant acid phosphatase, were negative for these cells. Flow cytometric immunophenotyping revealed that these lymphoid cells were significantly positive for CD19, CD20, and FMC-7, and negative for CD10, CD5, CD23, CD25, CD103, and surface immunoglobulins (SIg).
Conclusion: Based on these observations, SLVL was offered as a diagnosis. This case is also being reported to illustrate the diagnostic problems associated with this illness.
Keywords: SLVL; Lymphocytosis; Lymphoproliferative disorders; B-cell, Sudan
Introduction
Splenic Lymphoma with Villous Lymphocytes (SLVL) is a minimal B cell non-Hodgkin's lymphoma that has variable clinical and morphologic characteristics, such as splenomegaly without lymphadenopathy, lymphocytosis that rarely exceeds 100x 109/L [1]. WHO (2008) classification regarded it as the leukemic counterpart of Splenic Marginal Zone Lymphoma (SMZL), which accounts for less than 2% of lymphoid neoplasms [2,3]. Despite being less prevalent than other chronic lymphoproliferative disorders, SLVL is unrecognized maybe because there are few immunophenotyping tools and little public knowledge of it [4]. Accurate diagnosis of this condition is crucial as it differs in treatment and prognosis from common lymphoproliferative disorders [1]. In this case report, we present a case of SLVL that was initially misdiagnosed as Chronic Lymphocytic Leukemia (CLL) due to high total leukocyte counts and morphological appearance on light microscopy.
Case Presentation
A 67-year-old male sentry from Port Sudan was brought to our center in July 2022 as a case of CLL. For the past couple of years, the patient has experienced chronic exhaustion, some recurrent pruritic skin sores, and cramping. In addition, the patient had just lost a large amount of weight. On examination, the patient was found to have an 18 cm splenomegaly below the costal edge. There are no swollen lymph nodes in the peripheral area of the body. The laboratory test results revealed an absolute lymphocyte count of 72.6 x 109/L, a leukocyte count of 87 x 109/L, a hemoglobin level of 12.0 g/dl, and a platelet count of 131 x 109/L. The atypical lymphoid cells were morphologically larger than the tiny lymphocytes and possessed plenty of cytoplasm. Compact chromatin was present in the rounded nuclei. As opposed to CLL cells, there was less obvious chromatin clumping in these cells (Figure 1). Atypical lymphoid cells were found in the bone marrow aspirate at 39% and biopsy together with normal hematopoietic cells, and their villous appearance matched that of the peripheral smear. Venous blood was used for flow cytometric immunophenotyping, and the gated lymphocytes were strongly positive for CD19, CD20, and FMC-7 while being negative for CD10, CD5, CD23, CD25, CD103, and SIg. Studies on cytogenetics revealed a typical male karyotype.
The combination of clinical presentation, morphology, bone marrow involvement, and flow cytometry results strongly suggest a diagnosis of SLVL. It is crucial to thoroughly consider to achieve an accurate diagnosis and determine the most effective treatment plan; it is important to consider all of these factors.
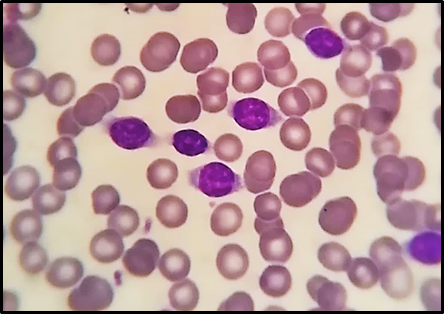
Figure 1: Villous lymphocytes are visible in the peripheral blood smear.
Discussion
SLVL is a low-grade B-cell lymphoma that Melo et al. [5] characterize in great detail. Marginal zone B-cell lymphomas are divided into three clinically different subgroups by the WHO classification system: SMZL, (extranodal marginal zone B-cell lymphomas of mucosa-associated lymphoid tissue) MALT lymphomas, and nodal marginal zone B-cell lymphomas [2,3]. SLVL is now recognized as the leukemic variant of SMZL in the most recent categorization [6].
It is an uncommon neoplasm, with only a few case series recorded in literature and making up fewer than 2% of all lymphoid neoplasms [4]. SLVL patients tend to be older than 50 and the frequency of the disease is equal for both sexes. Although it is believed that SLVL originates from B cells at an unknown stage of development, the presence of Ig gene somatic hypermutation in 50% of patients implies exposure to an antigen in the germinal center microenvironment [1].
Patients with SLVL typically have vague symptoms of anemia or pain in the left hypochondrium from splenomegaly. This frequently coexists with lymphocytosis. The SLVL cells are morphologically similar to small lymphocytes but slightly larger, with compacted chromatin, discrete nucleoli, a considerable amount of cytoplasm, and delicate villous projections on the cellular membrane. The presence of absolute lymphocytosis of more than 10 x109/l in the present case led to the first diagnosis of CLL. This matched the requirements of the international CLL workshop [7]. Only 15% of the lymphoid cells exhibited the distinctive morphological characteristics of SLVL. When they were first presented, these cells were disregarded. The first misinterpretation was caused by a very high lymphocytosis and the appearance of a small number of cells with delicate nucleoli and surface projections typical of SLVL [5].
Even though it has been observed, that high peripheral lymphocytosis is infrequent in SLVL; this is typically accompanied by moderate lymphocytosis [5]. This case strikingly lacked the distinctive checkerboard pattern of chromatin clumping seen in lymphoid cells of CLL. The lack of such lymphoid cells in a case should prompt the investigator to conduct a comprehensive morphological investigation and consider the possibility of a low-grade chronic lymphoproliferative disease other than CLL. The variable proportion of villous lymphocytes in SLVL cases presents a challenge for diagnosis based solely on appearance. [8] Care must be made to distinguish between the ruffled cytoplasmic boundaries of villous lymphocytes and the villous artifact on peripheral blood smears that result from smearing.
In the current patient, we found villous cells in 55% of all peripheral lymphocytes and 39% of the aspirated bone marrow. These lymphocytes had a round to oval nucleus with open chromatin, no nucleolus, and moderate cytoplasm with broad projections. In contrast to HCL, bone marrow displayed nodular infiltrate [9].
In the differential diagnosis of SLVL, it is important to keep in mind that other Chronic Lymphoproliferative Ailments, such as Prolymphocytic Leukemia (PLL), Hairy Cell Leukemia (HCL), Follicular Lymphoma (FL), and Mantle Cell Lymphoma (MCL), may display with overlapping clinical and morphological properties [1]. HCL cells have substantial villous projections on their surface, whereas PLL cells have moderate to copious amounts of cytoplasm. Follicular lymphoma (FL) leukemia is a type that is characterized by a predominance of cleaved cells. The differentiation from MCL cells could not be possible based solely on morphology, necessitating immunophenotyping [10]. Moreover, SLVL differs from HCL in that it does not exhibit tartrate-resistant acid phosphatase (TRAP) staining which is a distinguishing feature of HCL [11]. TRAP testing was negative in this case.
SLVL cells exhibit a diverse immunophenotypic antigen expression profile. A full panel of markers is needed as no single surface marker can distinguish SLVL from other lymphoproliferative illnesses. Mature B-cell markers (CD19 and CD20) were strongly positive in the current case, but the lack of CD5 and CD23 disqualified the diagnosis of CLL [1]. In our case, FMC-7 showed a significant rate of positive. The negative expression of CD25 and CD103 was beneficial in excluding HCL because FMC-7 is also substantially positive in HCL and HCL-V [1,4]. There is a significant overlap between the immunophenotypic profiles of SLVL and HCL-V. One marker that is positive in HCL-V but variable in SLVL is CD11c. Although the diagnosis cannot be validated purely based on immunophenotype, considering a holistic approach based on the clinical manifestation, appearance, bone marrow infiltration, and immunophenotypic profile, a diagnosis of SLVL above HCL-V was proposed [4].
Conclusion
Even with bone marrow infiltration, the clinical course of SLVL is indolent. Typical chemotherapy regimens, which are normally helpful in treating other chronic lymphoid neoplasms, are typically ineffective for these patients. It is crucial to diagnose this condition as a distinct entity since these patients often exhibit long-term survival after splenectomy.
Acknowledgments: "We are grateful for the help and cooperation of the patient and their family, and we express our sincere thanks to both"
Statement of Ethics: Reporting individual cases or case series is not subject to ethical review at our institution. The patient has signed a form approving the publication of this case report.
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Funding Sources: None
Author contribution: B.A.B. wrote the article and conducted the literature review, analysis, and reporting. The final manuscript was read by the author(s) and approved by them.
References
- Gupta R, Naseem S, Sukumaran S, Kashyap R, Kaur S, Paul Splenic lymphoma with villous lymphocytes. Indian J Pathol Microbiol, 2008; 51(1): 113-115.
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood, 2011; 117(19): 5019-5032. doi: 10.1182/blood-2011-01-293050.
- Bociek RG. Splenic marginal zone lymphoma: villous, not necessarily villainous. Oncology (Williston Park), 2012; 26: 208-210.
- Afrose R, Akram M, Khan NP, Rusia U. Splenic Lymphoma with Villous Lymphocytes: A Case Report with Review of Literature. Annals of Pathology and Laboratory Medicine, 2015; 2(3): C201-204.
- Melo JV, Hegde U, Parreira A, Thompson I, Lampert IA, Catovsky D. Splenic B cell lymphoma with circulating villous lymphocytes: Differential diagnosios of B cell leukaemias with large spleens. J clin pathol, 1987; 40: 642-651.
- Rebecca Sonu, Jeffrey Gregg, Mingyi Chen. Splenic marginal zone lymphoma with t (8;14) (q24.1; q32)/MYC rearrangement. J Hematopathol, 2012; 5: 141–147.
- International workshop on chronic lymphocytic leukemia guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood, 2018; 131(25): 2745-2760.
- Catovsky D, Matutes E. Splenic lymphoma with circulating villous lymphocytes/splenic marginal-zone lymphoma. Semin Hematol, 1999; 36:148-154.
- Cawley JC. The pathophysiology of the hairy cell. Hematol Oncol Clin North Am, 2006; 20: 1011-1021.
- Bain BJ. Chronic lymphoid leukaemias. In: Bain BJ, editors. Leukaemia diagnosis, 2 nd ed. Blackwell Science, 2017; p. 158-191.
- Bethel KJ, Sharpe RW. Pathology of hairy-cell leukaemia. Best Pract Res Clin Haematol, 2003; 16: 15-31.

