Pulmonary Alveolar Microlithiasis: The Stony Lung
Kenza Horache*, Kaoutar Imrani, Manal Jidal, Nabil Moatassim Billah and Ittimade Nassar
Radiology department, Ibn Sina University Hospital Center, Lamfadel Cherkaoui Street, Rabat, Morocco
Received Date: 02/05/2024; Published Date: 16/09/2024
*Corresponding author: Kenza Horache, Radiology department, Ibn Sina University Hospital Center, Lamfadel Cherkaoui Street, Rabat, MA 10170-Morocco
Abstract
Pulmonary alveolar microlithiasis is a rare diffuse lung disease distinguished by the presence by intra-alveolar microliths.
This condition arises from a mutation within the SLC34A2 gene, responsible for encoding the type IIb sodium phosphate cotransporter situated in alveolar type II cells.
During the early stages of the disease, patients typically remain asymptomatic and the pathology is usually detected incidentally. The clinical course varies, as it may persist stably in some individuals while progressing over time in others.
The radiological features on chest radiography include innumerable calcified micronodules that involve both lungs, predominantly in lower and mid zones. CT scan of the chest shows multiple sand-like calcifications throughout the pulmonary parenchyma. Additional features include “crazy paving” pattern, calcified interlobular thickening, and sub-pleural cysts.
Herein, we describe the case of a young adult who presented with complaints of progressive dyspnea, a nonproductive cough, and distinctive radiological manifestations consistent with pulmonary alveolar microlithiasis.
Keywords: Alveolar microlithiasis; Rare lung disease; Calcified micronodules; Crazy paving
Introduction
Pulmonary Alveolar Microlithiasis (PAM) was initially described by Friedrich in 1856 as an inherited pulmonary disorder. This condition is characterized by the diffuse pulmonary accumulation of microliths in the alveoli, contrasting with a paucity of symptoms.
The chest computer tomography scan confirms the presence of advanced microlithiasis that takes the form of calcified nodules, large ground-glass opacities, thickening, and calcification of interlobular septa.
In this report, we will study a case of PAM and conduct a comprehensive review of the literature pertaining to this disorder.
Case Report
A thirty years old male, without medical or toxic history, presented with progressively worsening dyspnea over the last 5 years associated with a non-productive cough.
The physical examination unveiled bilateral crepitation rales and hourglass nails. His oxygen saturation was 85% in room air.
The pulmonary function test revealed a restrictive ventilatory pattern.
He was referred to our radiology department for further investigations.
A chest radiograph (Figure 1) showed sand-like calcific micronodules involving both middle and lower areas of both lobes, with relative sparing of the apices.
The radiograph revealed that the calcific densities were confluent and denser at the bases.
Subsequently, a computed tomography of the chest was performed, showing bilateral large areas of ground-glass opacities associated with intra and interlobular septal thickening, calcified centrilobular nodules, and consolidation of the basal areas. Additionally, intra-alveolar calcifications were observed. However, there was no evidence of pleural or pericardial effusion.
These findings were highly suggestive of a Pulmonary Alveolar Microlithiasis (PAM).
Supportive treatment, involving oxygen therapy and corticosteroids, was administered, leading to symptomatic improvement. Regular follow-up appointments were scheduled for the patient.
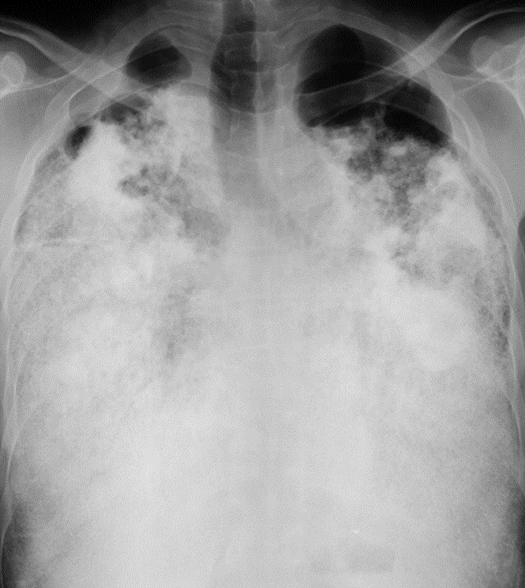
Figure 1: Chest x-rays demonstrates diffuse bilateral infiltrates with areas of dense micronodular, reticulation and dense consolidation mainly involving the lower zones.
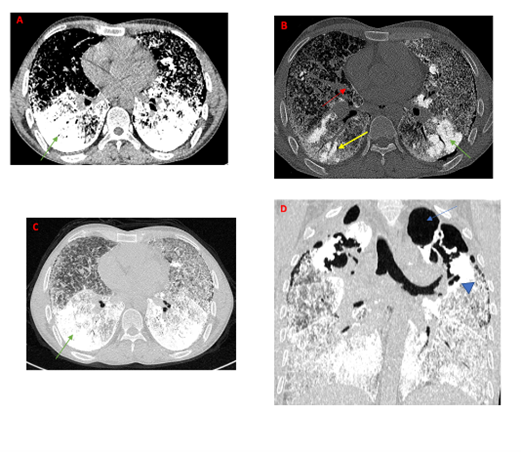
Figure 2: Computed tomography of the chest
A-C: axial view in mediastinum windowing (A), bone windowing (B) and lung windowing (C) demonstrating extremely dense calcic material at both lung bases (green arrow), intra-alveolar calcifications (yellow arrow), interlobular thickening and pleural calcification (red arrow)
D: coronal view lung window showing multiple sub-pleural cysts (head arrow) and apical bullae (blue arrow).
Discussion
Pulmonary alveolar microlithiasis represents a rare hereditary parenchymal lung disorder with no gender predilection. With fewer than 1000 reported cases [1], it is characterized by intra-alveolar calcopherites deposits made up of calcium and phosphorus [1-3].
Studies revealed a genetic background associated with the disease. PAM occurs due to the mutation of the SCLA34A2 gene, which encodes a type IIb sodium-dependent phosphate co-transporter in alveolar type II cells [2]. However, the etiology remains imprecise.
Typically, this condition onset occurs in individuals aged between 30 and 40 years old; with the exception of a few cases reported within a pediatric population [3].
The majority of patients remain asymptomatic, and the pathology is usually discovered incidentally during a chest radiography conducted for unrelated purposes [2].
Symptomatic patients, on the other hand suffer from dyspnea, chest pain, nonproductive cough and asthenia [2].
Pulmonary function assessments typically reveal either normal pulmonary function or a mildly restrictive pattern [1].
The disease normally progresses over a long period. For some patients, the symptoms remain stable, while in other cases, they could worsen over time, eventually leading to pulmonary fibrosis, respiratory failure or cor pulmonale.
Radiological findings will vary depending on the severity of the disease. On chest radiographs, PAM appears as a bilaterally diffuse areas of sand-like calcified micronodules described as “sandstorm”, predominantly observed in the middle and lower zones, accompanied by the black pleura sign [4].
On computed tomography of the chest, the appearance of the disease depends on the stage of the pathology.
Minimal changes can be detected, such as non-calcified micronodules in asymptomatic children.
The natural progression of the disease is accompanied by the apparition of multiple bilateral calcified micronodules, mainly involving the mid and lower areas.
As the disease develops further, the calcified micronodules increase in size and density, usually accompanied by ground-glass opacities, interlobular septal thickening, referred to as the “crazy-paving” pattern, with an appearance of a “stony lung”.
Additionally, sub-pleural cysts, apical bullae, and sub-pleural plaques are documented features in individuals diagnosed with PAM [4,5].
Differential diagnosis includes miliary tuberculosis and sarcoidosis, as well as pneumoconiosis, pulmonary amyloidosis and pulmonary alveolar proteinosis [1,3].
Since the SCLA34A2 gene is expressed in other tissues as well, mutations can cause extra-pulmonary calcifications leading to medullary nephrocalcinosis, nephrolithiasis, calcifications of the sympathic nervous system and possible testicular involvement [2,3].
Cardiac involvement has also been described including mitral stenosis, aortic valve stenosis, mitral, and aortic calcifications [2].
There is no known definite treatment for PAM, with the exception of lung transplantation considered the only effective treatment [2].
Some patients can show a good clinical response to steroid therapy. However, the effectiveness of corticosteroids is not established. Moreover, most authors agree that steroids cannot cure PAM [4,5].
Treatment with bisphosphonates can be proposed to children with PAM, as the chelate agents could eventually inhibit the development of microliths [2].
Conclusion
PAM is a rare genetic parenchymal lung disease characterized by intra-alveolar microliths.
The recognition of the typical radiological findings will help clinicians and radiologists to diagnose this unknown disease.
Competing interests: The authors declare that they have no competing interests.
References
- Al-Maghrabi H, Mokhtar G, Al-Maghrabi J, Meliti A. Pulmonary alveolar microlithiasis: A report of two unique cases. Respir Med Case Rep, 2019; 29: 100980. doi: 10.1016/j.rmcr.2019.100980.
- Jönsson ÅL, Simonsen U, Hilberg O, Bendstrup E. Pulmonary alveolar microlithiasis: two case reports and review of the literature. Eur Respir Rev, 2012; 21(125): 249-256. doi: 10.1183/09059180.00009411.
- Chu A, Shaharyar S, Chokshi B, et al. Pulmonary Alveolar Microlithiasis “Stone Lungs”: A Case of Clinico-Radiological Dissociation. Cureus, 2016; 8(8): e749. doi:10.7759/cureus.749.
- Ganesan N, Ambroise MM, Ramdas A, Kisku KH, Singh K, Varghese RG. Pulmonary alveolar microlithiasis: an interesting case report with systematic review of Indian literature. Front Med, 2015; 9(2): 229-238. doi: 10.1007/s11684-015-0394-y.
- Castellana G, Lamorgese V. Pulmonary alveolar microlithiasis. World cases and review of the literature. Respiration, 2003; 70(5): 549-55. doi: 10.1159/000074218.

