Intra-Abdominal Synovialosarcoma: A Case Report and Review of the Literature
Amine Bachar, Zakaria Essaidi, Malik Baallal Zarhouni*, Taoufik El Abbassi and Mohamed Rachid Lefriyekh
Department of Medicine, Hassan 2 University Faculty of Medicine, Morocco
Received Date: 03/04/2024; Published Date: 11/09/2024
*Corresponding author: Malik Baallal Zarhouni, Department of Medicine, Hassan 2 University Faculty of Medicine, Morocco
Abstract
Primary abdominal synovial sarcoma is a rare mesenchymal tumor, which presents as a slow-growing intra-abdominal mass, having a heterogeneous appearance with eccentric calcifications. The anatomopathological study of the biopsy or the surgical specimen makes it possible to confirm the diagnosis.
The standard treatment is “wide excision” surgery. However, the risk of recurrence remains high, which is why early diagnosis is optimal.
Introduction
Synovialosarcoma is a primary malignant mesenchymal tumor first described in 1893, which accounts for 2.5 - 10.5% of all primary soft tissue cancers [1].
Synovial sarcoma is considered to be a tumor arising from the synovium following par articular localization and histological similarities with synovial tissue. This explains where the name comes from: synovial sarcoma.
However, synovial sarcoma is not linked to synovial tissues, it is made up of dedifferentiated mesenchymal cells, this tumor proliferation is associated with the chromosomal translocation t (X; 18) (p11; q11) [2-4].
Synovial sarcoma develops mainly in the legs, arms and knees, but it can appear with a percentage of 5 to 10% in the head, neck, mediastinum, abdominal wall, oesophagus, and retro peritoneum [5] Other unusual locations have been reported such as: the skin, blood vessels, nerves, mediastinum, pleural cavity, prostate, kidney [5], digestive tract, liver, and the aero digestive crossroads [6-10].
Primary intra-abdominal synovial sarcoma has been mentioned as a rare case in several reports. [11-13].
In this article we will discuss a case of a 38-year-old female patient who presented with an abdomino-pelvic mass gradually increasing in size, and which ultimately turned out to be a biphasic intra-abdominal synovial sarcoma after surgical excision.
Clinical Observation
This is a 38-year-old patient, with no notable medical or family history, who has presented since May 2020 with a progressive increase in the volume of the abdomen, painless without vomiting, nor occlusive syndrome, nor associated digestive haemorrhages, all evolving in a context of conservation of the general condition with the clinical examination we find a patient in good general condition ps:0 abdominal examination objective a flexible non-distended abdomen, a peri-umbical mass, 10 cm long axis painless and mobile in relation to the plane superficial and deep rectal and vaginal examination normal, and the rest of the somatic examination is unremarkable.
An abdominal and pelvic CT scan from 05/30/21: Intraperitoneal mass in the peri-umbilical region, solid, cystic with a tumoral appearance which measures 73*5Smm extended over 79mm in front with the anterior abdominal wall with persistence of fatty border of separation, laterally it comes into contact with the small intestine without fatty border of separation
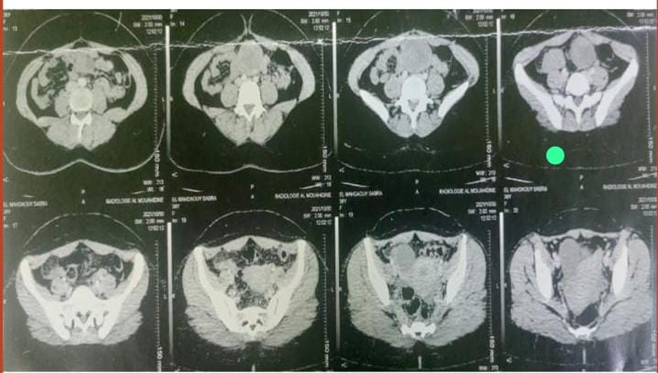
Figure 1: CT image showing the periumbilical mass.
The patient was operated on 06/21/21 in our department, she had a segmental small bowel resection of 15 cm at 1M60 from duedeno jéjunal angle with end to end grelic anastomosis , surgical exploration revealed a very abundant hematic peritoneal effusion with a grelic mass at 1M60 from the duedeno jéjunal angle measuring 10 cm long axis with solid cystic component and site of rearrangement hemorrhagic necrotic The post-operative consequences were simple and the patient was declared discharged on D5 post-operative.
The anapath of the operating specimen: Malignant tumor proliferation poorly differentiated biphasic, Spindly wall is respected and without characterized lesion. The hail limits are healthy. Absence of ON+/4N lymph node metastasis.
The long-term evolution was marked one year after his intervention by the appearance of a pelvic mass gradually increasing in painless volume without other associated signs in a context of preservation of the general condition with the clinical examination, we found still a patient in good general condition PS 0, The abdominal examination notes a flexible, non-distended abdomen with a central pelvic mass of 5 cm mobile in relation to the 2 planes
A TAP CT was requested on 02/07/23: Presence of a well-limited heterodense left subphrenic lesion process, with regular contours, with a predominant cystic component, seat of a fleshy portion and thick septa, enhanced after injection of PDC. It measures 86.5x53.5mm, extended to 82mm.
We also note the presence of a second centro-pelvic process of multiloculated fluid density seat of fine closures enhanced after injection of PDC measuring 158x75.6 mm extended over 124 mm. It is associated with another right latero-uterine formation in continuity with the process described above. Bilateral external iliac lymphadenopathy, the largest is right Compared to the pre-operative examination of 10/30/2021, we note: CT appearance in favor of a double intra-peritoneal tumor process under left phrenic and pelvic in favor of a recurrence probable tumor. Absence of clearly detectable secondary lesion.

Figure 2: Scanographic image showing the pelvic mass.
The patient's file was assigned to CPR and the decision was to make a surgical cessation:
The patient was operated on 03/09/23 by a median laparotomy, the surgical exploration revealed the presence of a very abundant fluid effusion made of hematic fluid with the presence of 2 lunar cystic masses at the sub phrenic level left measuring 11 cm long axis and the other at pelvic level measuring 19 cm long axis The surgical procedure consisted of a total excision of the two cystic masses The post-operative consequences were simple and the patient was declared discharged on D3 after resumption of transit.
Ana path of the surgical specimens confirmed the recurrence of the Synovial sarcoma already known in the patient The patient was subsequently lost to follow-up
Discussion
Etiology and demography Synovial sarcoma is a misnomer which represents a tumor that only resembles synovial tissue under an optical microscope, and which originates from multipotent stem cells capable of differentiating into mesenchymal and/or epithelial structures, which explains the different extra-articular locations [14-19].
Synovial sarcoma occurs most commonly in young people, accounting for approximately 5-10% of all soft tissue sarcomas, but approximately 15-20% of cases occur in adolescents and young adults with an estimated incidence of 2.5 per 100,000 [15,20,21].
Although the most common location of synovial sarcoma is the particular region (close to large joints), particularly the joint capsules, tendon sheaths, and bursae, other unusual extra-articular locations have been reported in the literature such as: the abdominal wall, abdominal cavity, retro peritoneum, head, cervical region, lungs, mediastinum, pleura, heart, kidney, prostate, vessels, nerves... [5-10].
Clinical, radiological, and pathological study Primary abdominal synovial sarcoma most often presents in the form of a painless, palpable, slowly growing mass, whose clinical symptoms (especially signs of compression) depend on the site and the size of the tumor. Metastases occur in the first 2 to 5 years, they are present in 16-25% of cases, particularly in the lungs, and less frequently in the lymph nodes and bones [19].
Even if imaging does not confirm the diagnosis, it can be suspected in the presence of a heterogeneous mass with necrotico-hemorrhagic areas and eccentric or peripheral calcifications. These calcifications are present in 30% of cases [22,23]. CT scanning is more sensitive than abdominal ultrasound because it can detect calcifications as well as necrosis and haemorrhagic areas.
Regarding magnetic resonance (|MR) imaging, synovial sarcoma appears isointense to slightly hyperintense on T1-weighted images, and hyperintense to muscles on T2-weighted images due to the presence of a mixture of solid, cystic, fibrous and myxoid stromal tissues.
Marked heterogeneity and enhancement are strongly suggestive of synovial sarcoma, as are CT findings [22,23].
The main differential diagnoses of intra-abdominal synovial sarcoma are fibro sarcomas, malignant schwannomas, malignant fibrous histiocytomas. Histological study after a biopsy or surgical resection represents the only way to confirm the diagnosis of synovial sarcoma. The most sensitive immunohistochemical stains for synovial sarcoma are EMA, cytokeratin AE1/AE3 and E-cadherin, in association with CD 34 negative [17].
Keratin positivity (around 90% of cases), measured by immunostaining associated with the histological appearance, confirms the positive diagnosis of a synovial sarcoma [1]. Synovial sarcoma has three histological subtypes: monphasic, biphasic and the poorly differentiated type [1].
The monophasic subtype accounts for 50-60% (the most common subtype) of all lesions, it consists mainly of spindle cells resembling fibrosarcoma, while the biphasic subtype is composed of a bimorphic form, with mixed spindle and epithelioid cell components [1,18]. Concerning the poorly differentiated subtype, it is generally of epithelioid morphology associated with geographic necrosis with high mitotic activity (15-20/10 field). This subtype represents 15 to 25% of all synovialsarcomas [1].
Genetic background: The specific chromosomal aberrations are the translocation (X;18) (p11.2:q11.2) found in 90% of SS and the fusion of the SYT gene on chromosome 18 with either SSX1 (67% of cases) or SSX2 (33% of cases) on chromosome X [19 ].
Tumors of monophasic subtypes mainly express the SYT-SSX2 fusion transcript while biphasic tumors mainly carry the SYT-SSX1 transcript [17].
Treatment and prognosis: Wide surgical excision remains the only curative treatment. For non-metastatic tumor of small size < 5cm, wide surgical excision is indicated, whereas for large, locally advanced, metastatic tumor, neoadjuvant chemotherapy seems indicated to reduce the tumor volume and thus allows in 61 to 80% of cases a surgical procedure that was initially impossible [24-26].
Adjuvant chemotherapy aims to reduce the rate of recurrence and the incidence of metastases [24, 25].
However, recidivism still varies between 28% and 36% [18].
Close follow-up of at least every 3 months for the first 2 years then semi-annually for an additional 5 years is recommended due to the high local recurrence [19].
Survival and prognosis are correlated with tumor size, histological subtype, mitotic rate, glandularity percentage, tumor necrosis and vascular invasion [26]. The poorly differentiated subtype includes epithelioid cells with high mitotic activity which explains the high grade (3/3). Monophasic and biphasic synovial sarcomas are generally intermediate grade (2/3) [18].
Conclusion
Primary intra-abdominal synovial sarcoma is an aggressive, rare tumor, known for its high recurrence and mortality rate. Consequently, it must be considered in the face of any aggressive abdominal mass because the prognosis depends on early management.
References
- Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics, 2006; 26(5): 1543–1565; Int. J. Adv. Res. 9(08), 89-95
- Limon J Mrokek, Nedozeytko B, Babinskai M, Jaskiewing J, Kopacz A, Zoltowski A, et al. Cytogenetic findings in two syovial sarcomas. Cancer Genet. Cytogenet, 1989; 38: 215-222.
- Noguera R, Lopez- Gines C, Gill R, Carda C, Pelina, Llombardbos Ch A. Translocation (18) in a synovial sarcoma a new case.Cancer Genet C ytogenet, 1988; 33: 311-312.
- Mandahl N, Heims S, Arheden K , Kydholma A, Willen H, Mitelman F. Multiple cariotypic rearrangements, including t (18) (P11,q11) in a fibro sarcoma. Cancer genet. Cytogenet, 1988; 70A: 1561-1567.
- Wang YJ, Wen SC, Chien ST, Sheu JW, Hsuea CW, Feng NH. Primary intra-abdominal synovial sarcoma. J Chin Med Assoc, 2006; 69(10): 492–495.
- Billings SD, Meisner LF, Cummings OW, Tejada E. Synovial sarcoma of the upper digestive tract: a report of two cases with demonstration of the X;18 translocation by fluorescence in situ hybridization. Mod Pathol, 2000; 13: 68-76.
- Schreiber-Facklam H, Bode-LesniewskaB, Frigerio S, Flury R. Primary monophasic synovialsarcoma of the duodenum with SYT/SSX2 type of translocation. Hum Pathol, 2007; 38: 946-949.
- Makhlouf HR, Ahrens W, Agarwal B, Dow N, Marshalleck JJ, Lee EL, et al. Synovial sarcomaof the stomach: a clinicopathologic, immunohistochemical,and molecular geneticstudy of 10 cases. Am J Surg Pathol, 2008; 32: 275-281.
- Dei Tos AP, Dal Cin P, Sciot R, Furlanetto A, DaMosto MC, Giannini C, et al. Synovial sarcoma of the larynx and hypopharynx. Ann Otol Rhinol Laryngol, 1998; 107: 1080-1085.
- Srivastava A, Nielsen PG, Dal Cin P, Rosenberg AE. Monophasic synovial sarcomaof the liver. Arch Pathol Lab Med, 2005; 129:1047-1049.
- Ko SF, Chou FF, Huang CH, Ng SH, Wan YL, Lee TY, et al. Primary synovial sarcoma of the gastrocolic ligament. Br J Radiol, 1998; 71: 438-440.
- Hewavisenthi SJ, Collure SK. Synovial sarcoma is an unusual site. Ceylon Med J, 2000; 45: 82-83.
- Spillane AJ, A’Hern R, Judson IR, Fisher C, Thomas JM. Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol, 2000; 18: 3794-3803.
- Braham E, Aloui S, Aouadi S, Drira I, Kilani T, El Mezni F. Synovial sarcoma of the chest wall: a case report and literature review. Ann Transl Med, 2013; 1(1).
- Karadag O, Altundag K, Elkiran ET, Dikbas O, Gedikoglu G, Kars A. Anterior abdominal wall synovial sarcoma: a rare presentation. Am J Clin Oncol, 2005; 28(3): 323-324.
- Hale JE, Calder IM. Synovial sarcoma of the abdominal wall. Br J Cancer, 1970; 24(3): 471-474.
- Vera J, García MD, Marigil M, Abascal M, Lopez JI, Ligorred L. Biphasic synovial sarcoma of the abdominal wall. Virchows Arch, 2006; 449(3): 367-372.
- Saif AH. Primary synovial sarcoma of the abdominal wall: a case report and review of the literature. J Family Community Med, 2008; 15(3): 123-125.
- Jayaraman S, Rao SD, Govindarajan M. Synovial sarcoma of anterior abdominal wall. Indian J Surg, 2010; 72(Suppl 1): 293-295.
- Weiss SW, Goldblum JR. Enzinger and Weiss’s soft tissue tumors. Mosby. Inc. A Harcourt Health Sciences Company, St Loui, 2001.
- Hampole MK, Jackson BA. Analysis of 25 cases of malignant synovioma.Canmed.Assoc.j, 1968; 99: 102561029.
- Bakri A, Shinagare AB, Krajewski KM, Howard SA, Jagannathan JP, Hornick JL, et al. Synovial sarcoma: imaging features of common and uncommon primary sites, metastatic patterns, and treatment response.AmJRoentgenol, 2012; 199(2): W208–W215.
- Kishino T, Morii T, Mochizuki K, et al. Unusual sonographic appearance of synovial sarcoma of the anterior abdominal wall. J Clin Ultrasound, 2009; 37(4): 233-235.
- Mahfoud M. Cancer de l’os (tumeursMalignes des membres). Edition, 2009.
- But B, Blay J, Bonichon F, Morice P, Raycoqvard I, Taieb S, et al. Recommendations for the management of adult patients with soft tissue sarcomas, 2006.
- Antman KH, Ryan JR, Baker LO. Chemotherapy of advanced soft tissu sarcomas. Ends recent concept in sarcomas treatment. Dodrecht, 2023.

