Malignant Testicular and Paratesticular Tumors in Children
Manni A1,2,*, Zouirech Y1,2, Rouijel B1,2, Hadir M1,2, Bouljrouf J1,2, Ouchen M1,2, Hallout M1,2, Zerhouni H1,2 and Kisra M1,2
1Department of Pediatric Surgery 'A', Rabat Children's Hospital, Morocco
2Faculty of Medicine and Pharmacy, Mohammed V University, Rabat, Morocco
Received Date: 06/04/2024; Published Date: 29/08/2024
*Corresponding author: Abir Manni, Department of Pediatric Surgery 'A', Rabat Children's Hospital, Morocco; Faculty of Medicine and Pharmacy, Mohammed V University, Rabat, Morocco
Abstract
Introduction: Testicular tumors (TTs) are rare in children, and their prognosis remains excellent despite the risk of locoregional and general progression. The importance of the initial secretion of tumor markers, the presence of metastases, and the quality of surgical excision are important prognostic factors in defining different therapeutic groups.
Materials and methods: A retrospective study of 7 TT cases in children under 15 years of age treated in our center from 2015 to 2021 was carried out.
Results: The mean age of the patients was 2.9 years (range: 1- 8.8 years). The peak was in the first 2 years (5 cases). All patients presented with an asymptomatic scrotal mass. The right testicle was the most affected (5 cases). The α-Fetoprotein levels were elevated in 5 patients. The histological study showed 5 non-seminomatous germ cell tumors (71.4%): 2 yolk sac tumors (28.5%), 2 mixed germ cell tumors (28.5%),1 embryonal carcinoma (14.2%) and 2 para-testicular rhabdomyosarcomas (28.5%). In all cases, the surgical approach was a radical inguinal orchiectomy. The tumors were stage I in 4 patients and stage II in 3 patients. Chemotherapy was applied in all cases, preoperative (3 cases), postoperative (3 cases), and both (1 case). The survival rate was 86% at 5-year follow-up for all stages.
We emphasize this study not just for the clinical presentation, diagnosis, and therapy of these children, but also to raise clinician knowledge of this rare type of malignancy.
Keywords: Testicular tumor; Children, histology; Inguinal approach; Orchiectomy
Abbreviations: TT: Testicular Tumor; AFP: Alpha-Fetoprotein; β-HCG: A beta subunit of Human Chorionic Gonadotropin; LDH: Lactate Dehydrogenase; US: Ultrasonography; CT: Computed tomography; YST: Yolk Sac Tumor; RMS: Rhabdomyosarcomas; MR: Magnetic Resonance; WHO: World Health Organization
Introduction
Testicular and paratesticular tumors are rare in children under 15, then accounting for 2–4% of all childhood cancers [1,2]. They have two peaks of incidence in the pediatric population: neonatal and puberty [3]. These tumors have a favorable prognosis, with a benignity rate of almost 50% and a 5-year overall survival rate of 99% [4].
The majority of patients are asymptomatic at the time of diagnosis. Physical examination, imaging studies (particularly ultrasound imaging), and tumor markers contribute to the diagnosis [5]. The most commonly used diagnostic and follow-up indicators are (AFP) and beta subunit of human chorionic gonadotropin (β-HCG).
Radical orchiectomy is widely recognized as the gold standard treatment for testicular masses in all age groups [6]. Chemotherapy with or without radiotherapy is added to surgical treatment for high-risk or aggressive histologic forms, but due to the benign nature of the majority of these tumors, the therapeutic strategy has been revised and testicle-sparing surgery has gained prominence [7,8].
Materials and Methods
A prospective descriptive study from January 2015 to December 2021 has been carried out. Included are children under 15 years of age managed for testicular or para-testicular tumors in our center. We recorded and analyzed medical data, and patients were followed up for a mean of 5.8 years.
We excluded patients with an underlying risk of testicular tumors, testicular tumors discovered in children who underwent orchiectomy for undescended atrophic testis, and all secondary tumors, mostly metastases from lymphomas and leukemias.
the variables analyzed were age at diagnosis, presenting complaints, associated symptoms, laterality, clinical and radiological findings, tumor markers, histological type, management, recurrence, and follow-up.
After clinical evaluation, all patients underwent testicular ultrasonography (US) and serum levels of tumor markers (AFP and β-HCG). In addition, chest X-rays, and abdominal-pelvic Computed Tomography (CT) scans were performed to look for metastases.
The surgical approach was radical inguinal orchiectomy in all cases. Adjuvant treatment (chemotherapy and/or radiotherapy) was administered selectively based on histologic findings and according to international recommendations for treating TTs in children.
Follow-up focused on clinical examination, monitoring tumor markers, and imaging findings (CT scan, MR, bone scintigraphy). Data were collected and analyzed using Epi Info 7.6, with strict adherence to ethical principles.
Results
The study group included 7 children. The mean age was 2.9 years [1 year- 8.8 years], and 5 patients were ≤ 2 years, while 2 were > 2 years. No cancer history was recorded. Only one patient had contralateral cryptorchidism. The average time between symptom onset and consultation was 4 months (range 1 and 9 months). All patients presented with an asymptomatic scrotal mass (Figure 1A), which increased progressively in 6 cases (86%) and rapidly in 1 case (14%). The right testicle was the most affected in 5 out of 7 cases.
There were 5 TTs and 2 Para-Testicular Tumors (PTTs). 5 right-sided and 2 left-sided, and none of our patients had signs of puberty. The US revealed testicular and paratesticular lesions (Figure 2), The tumor appeared as a heterogeneous mass with hypoechoic echostructure vascularized on Doppler, and containing microcysts in 1 case. Hydrocele was detected in 1 patient, while spermatic cord thickening was observed in 1 patient. Tumor sizes ranged from 15 to 41 mm in diameter.
AFP levels were higher than the physiologic values in 5 patients (71.4%) (non-seminomatous germ cell tumors), among them, the value was > 15000 ng/ml in 4 patients. β-HCG was negative in all our patients. LDH was requested in 3 patients, one of whom was positive.
The anatomopathological study of the 7 cases studied confirmed the diagnosis of 5 primary TTs and 2 PTTs. 5 non-seminomatous germ cell tumors (71.4%): 2 Yolk Sac Tumors (YST) (28.5%), 2 mixed germ cell tumors (28.5%) and 1 embryonal carcinoma (14.2%). The 2 PTTs were embryonal rhabdomyosarcomas (RMS) (28.5%). In 4 of the cases (57.2%) the tumor was in stage I and in 3 cases (42.8%) stage III, according to the staging system for malignant testicular and paratesticular tumors of the Children's Cancer Group and the Pediatric Oncology Group (Table 1) [23]. The 3 cases of disseminated disease were 1 embryonal carcinoma and 2 rhabdomyosarcomas, all of them presenting retroperitoneal metastases > 2 cm.
In all cases, the surgical approach was radical inguinal orchiectomy, exteriorizing the testicle with a high and atraumatic clamp of the spermatic cord to prevent bleeding and tumor dissemination (Figure 1B). No conservative treatment was performed on our patients. An intraoperative biopsy confirmed the diagnosis of YST in one patient. Lymph node dissection was performed in 3 patients (42.8%), 1 in the inguinal region and 2 in the iliac region.
In addition to surgical treatment, all patients received adjuvant chemotherapy: preoperative (3 cases), postoperative (3 cases), and both (1 case). It was the RMS 2005 protocol for rhabdomyosarcoma and the TGM 95 protocol for YST.
Postoperatively, one patient (RMS) who did not receive chemotherapy initially, developed a local recurrence and was treated with chemotherapy followed by a second tumor excision along with hemicortectomy. Another case (YST) was lost to follow-up after radical orchiectomy but later presented with lung metastases. After one year, the patient was treated with 7 cures of chemotherapy followed by pulmonary metastasectomy.
During follow-up, US examinations and serum AFP measurements were performed at month 1, month 3, month 6, and then annually. One patient has abandoned the follow-up since 2017, all the other patients were alive and disease-free at their last outpatient checkup. The median follow-up was 70 months. Considering all stages, the 5-year overall survival rate for all patients was 86%.
Table 1: Staging system of testicular and paratesticular malignant tumors (Children’s Cancer Group and Pediatric Oncology Group) [23].
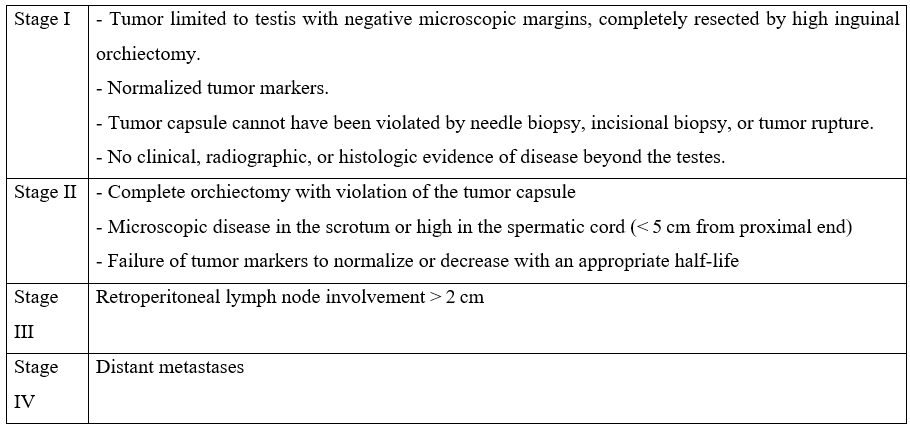
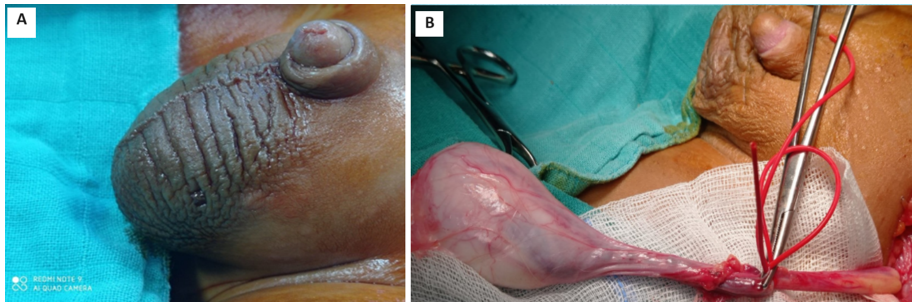
Figure 1: A: Left-sided scrotal mass seen in a prepubertal boy. B: Intraoperative image of the patient undergoing an inguinal radical orchiectomy.
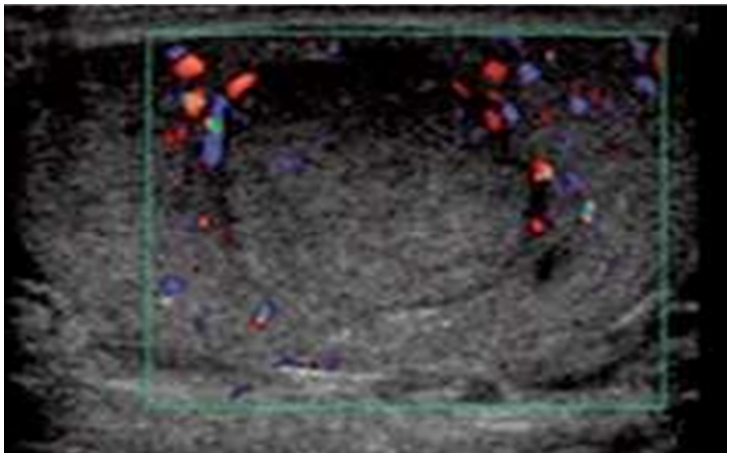
Figure 2: Longitudinal ultrasound view of the left testis shows hypoechoic mass (Embryonal carcinoma).
Discussion
TTs and PTTs are a very rare pathology in the pediatric population [1], and occur at an incidence of 0.5 to 2/100,000 children [2,8]. In our institution, 7 cases were recorded over 7 years. They have a bimodal distribution: the first peak is seen in boys < 4 years of age, most being benign, and the second occurs after puberty, from age 15 to 19 years, with a higher proportion of malignant neoplasms [3]. The mean age of our patients was 2.9 years similar to the literature. This observation highlights two distinct patterns of disease leading to different approaches to treatment.
Various risk factors for TT development in all ages have been described including a history of cryptorchidism, Klinefelter syndrome, a history of testicle cancer in first-degree relatives, and the presence of contralateral tumor [5]. There were no risk factors in our series, one patient had contralateral cryptorchidism.
TTs usually present as a painless testicular mass in 82 to 90% of cases and as a painful mass secondary to hemorrhage or necrosis in less than 10% [2]. In our study, all cases presented as an asymptomatic palpable mass, consistent with findings from other studies [2,3,6,9]. Physical exploration reveals the presence of a solid mass of stony consistency, without signs of inflammation. Hydrocele exists in 15-50% of TTs [8,10,11]. In our study one patient had hydrocele ipsilateral. The examination is essential in the differential diagnosis of other pathologies of traumatic or inflammatory origin, such as testicular torsions, epididymoorchitis, hydroceles, or inguinal hernias. Some TTs are accompanied by precocious puberty or gynecomastia [12]. However, this was not the case in our series.
The evaluation of TTs in a child will be similar to that of adults, using testicular US and tumor markers: AFP and β-HCG [8]. The US has almost 100% sensitivity in detecting TTs, with a low specificity of 75% because the differentiation between benign and malignant neoplasms is difficult in most cases [2,13]. TTs are predominantly homogeneous hypoechoic, but can also be heterogeneous with solid, cystic, or calcific components that reflect the underlying histologic characteristics [14]. Doppler US can be useful for diagnosis, and assessing a possible increase in vascular flow [2,10]. Benign tumors will appear as well-defined masses, with delimited edges and poor vascularization. Epidermoid cysts appear as well-defined intratesticular lesions, with a central hypoechoic area surrounded by a hyperechoic halo. YST has a more solid, hypoechoic, and homogeneous appearance [10]. Magnetic Resonance (MR) can be necessary in rare cases where scrotal US findings are inconclusive or non-diagnostic.
Tumor markers are useful for the diagnosis and treatment of metastatic disease progression. The most important marker is AFP is elevated in over 80-90% of YST and 30.4% for immature teratomas [9]. The ß-HCG is high at 2.2% for YST and 2.7% for immature teratomas [15]. In our cases, the AFP was high in 5 cases (non-seminomatous germ cell tumors) and β -HCG in 3 cases. Testosterone levels could be elevated in Leydig cell tumors [12]. Lactate dehydrogenase (LDH) is not very specific but will appear elevated especially in patients with metastatic disease, as a marker of tissue destruction [8]. In North American and European protocols, tumor marker negativity is an indispensable criterion for considering conservative surgery [10,16].
According to the World Health Organization (WHO), TTs are divided into seven groups (Table 2). A pathogenetic differentiation between two types of yolk sack tumors and teratomas is carried out: postpubertal, which are derived from a precursor lesion (germ cell tumors derived from germ cell neoplasia in situ—GCNIS), and prepubertal, (unrelated or non-GCNIS) [17].
Table 2: The 2016 WHO classification of testicular tumors [17].
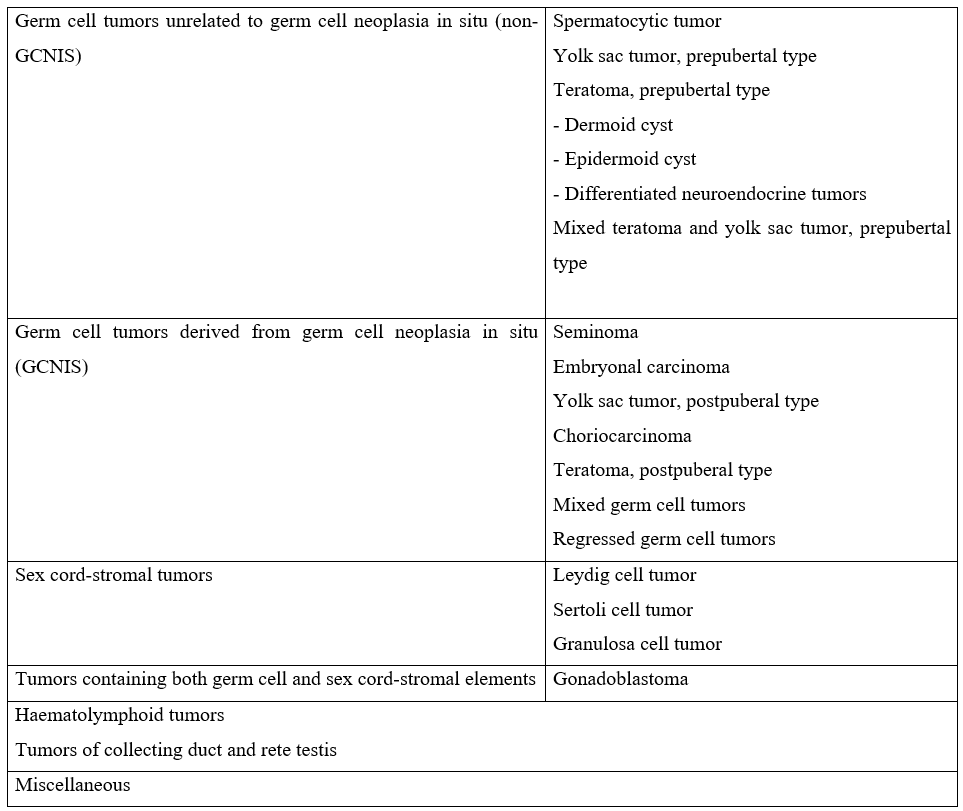
Regarding histology, the literature demonstrates a higher prevalence of germ cell tumors: Prepubertal teratomas (50%), Prepubertal yolk sac tumors (15%), and epidermoid cysts (15%). Other tumors are epidermoid cysts (15%) and stromal tumors (Leydig cell and Sertoli cell), which account for approximately 10% [3,11]. Some series describe yolk sac tumors as the most frequent because benign tumors are not included [2]. Mixed germ cell tumors rarely occur in prepuberal boys while in postpuberal boys most tumors are malignant and mixed germ cell tumors are present in greater numbers [11,18].
RMS is the most common malignant PTT (40%), accounting for 5% of all malignant testicular and paratesticular tumors [19]. It is the most prevalent spermatic cord sarcoma. Paratesticular RMS represents 7% of all RMS [20]. Patients with paratesticular RMS typically present with two peaks, one at the age of 4 years and the second at the age of 18 years [21]. According to Taskinen et al., 68% of pre-pubertal patients have a benign tumor, this proportion decreases to 38% after puberty [22]. In the series of cases presented, malignant tumors predominate, although we must take into account the possibility that not all benign testicular masses have been recorded, as occurs in other series published.
Malignant germ cell tumors spread first via lymphatic, usually through the inguinal ring and spermatic cord to the retroperitoneum, which only occurs in 4-6% [11]. Haematogenous spread occurs in 61% of adults and only in 9% of children, with the lungs being the most common site (in 20% of yolk sac tumors) [9]. In our series, 3 patients (42.8%) had retroperitoneal metastases and 1 case (YST) developed lung metastases.
TTs have staging systems based on pathology after orchiectomy or tumorectomy, radiology with a chest x-ray, MR or thoracoabdominopelvic scan, and serum tumor markers (Table 1) [23]. In our series 4 cases were stage I (57.2%) and 3 cases were stage III (42.8%), The 3 cases of disseminated disease were 1 embryonal carcinoma and 2 rhabdomyosarcomas, all of them presenting retroperitoneal metastases > 2 cm.
Needle biopsy of testicular masses is not indicated, since this technique could favor tumor dissemination [8]. TTs should be considered malignant until proven otherwise [24]. In cases of suspected testicular tumors, the inguinal approach is preferred over the scrotal approach to avoid scrotal violation. Additionally, frozen section examination (FSE) may improve diagnostic accuracy, particularly in doubtful cases, and can support an organ-sparing approach [8,25]. In our study FSE was performed in one patient and confirmed the diagnosis of YST.
Testicular-sparing surgery is the preferred treatment for benign tumors. Radical inguinal orchiectomy is indicated when a malignant tumor is identified [26,27]. Inguinal orchiectomy remains the gold standard in our institution. Chemotherapy is selectively indicated following the histologic founding.
- For rhabdomyosarcoma, polychemotherapy is indicated for 18 to 24 months. These protocols are VAC, IVA, and VIE (V: vincristine; A: actinomycin D; E: Etoposide; I: ifosfamide; C: cyclophosphamide) [28]. The IVA was the one done for our patients.
- The TG M95 protocol and the association VIP (Etoposide, ifosfamide, and cisplatin) were used for the Yolk sac tumor.
The local recurrence and metastases are the risk for testicular and para-testicular tumors in children [29]. These complications were noticed in 2 patients. After conservative surgery, the rate of recurrence varies from 1.1 to 5%, and testicular atrophy from 0 et 0.37% [29,30].
Mortality in childhood is low, with prepubertal testicular cancer having a survival rate of 99% at 5 years [4,10]. No case of death was reported; one patient was lost for follow-up. The 5-year overall survival rate for all of our patients was 86% in all stages.
Conclusion
Testicular and paratesticular tumors represent rare entities in childhood and adolescence, their knowledge is of great relevance for an adequate diagnosis and treatment, given their histological, evolutionary, and therapeutic characteristics, well-differentiated concerning those found in postpubertal patients or adults. Its low incidence makes universal therapeutic guides difficult to obtain and, in many cases, certain therapeutic aspects are still analyzed from the experience of different groups with non-comparable individualized treatments.
Author Contributions: All authors contributed to the writing of this manuscript, and all read and approved the final version
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Consent to publish the case report was not obtained. This report does not contain any personal information that could identify the patients.
References
- Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol, 2017; 18: 719–731.
- Caballero Mora FJ, Muñoz Calvo MT, García Ros M, et al. Testicular and paratesticular tumors during childhood and adolescence. A Pediatr (Barc), 2013; 78: 6–13.
- Romo Muñoz Martha Isabel, Núñez Cerezo Vanesa, Dore Reyes Mariela, Vilanova Sánchez Alejandra, González-Peramato Pilar, López Pereira Pedro, et al. Testicular tumors in children: Indications for testis-sparing surgery. Anales de Pediatría (English Edition), 2018; 88(5): 253–258. doi: 10.1016/j.anpede.2017.05.009.
- Trama A, Mallone S, Nicolai N, et al. The burden of testicular, para testicular and extragonadal germ cell tumors in Europe. Eur J Cancer, 2012; 48: 159-69. doi: 10.1016/j.ejca.2011.08.020.
- Walsh TJ, Grady RW, Porter MP, Lin DW, Weiss NS. Incidence of testicular germ cell cancers in U.S. children: SEER program experience 1973 to 2000. Urology, 2006; 68: 402–405.
- Wang W, Sun Z, Chen Y, Zhao F, Yu H, Guo X, et al. Testicular tumors: Discriminative value of conventional MRI and diffusion-weighted imaging. Medicine, 2021; 100: e27799.
- Murcia-Pascual FJ, Gracia-Rodríguez R, Vázquez-Rueda F, et al. Tumores testiculares y paratesticulares en la edad pediátrica. Arch Esp Urol, 2016; 69(10): 691–697.
- Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, et al. Guidelines on Testicular Cancer: 2015 Update. Urol, 2015; 68: 1054–1068. doi: 10.1016/j.eururo.2015.07.044.
- Bujons A, Sfulcini JC, Pascual M, Feu OA, Garat JM, Villavicencio H. Prepubertal testicular tumors and efficacy of testicular preserving surgery. Br. J. Urol, 2011; 107: 1812–1816. doi: 10.1111/j.1464-410X.2010.09796.x.
- Ahmed HU, Arya M, Muneer A, Mushtaq I, Sebire NJ. Testicular and paratesticular tumors in the prepubertal population. Lancet Oncol, 2010; 11(5): 476–483. doi: 10.1016/S1470-2045(10)70012-7.
- Britos A, Scivoli F, Gutiérrez C, Castillo L, Chavarria O. Primary testicular tumors in children. Rev Cir Infant, 2000; 10: pp. 196-200.
- Metcalfe PD, Farivar-Mohseni H, Farhat W, McLorie G, Khoury A, Bägli DJ. Pediatric testicular tumors: Contemporary incidence and efficacy of testicular preserving surgery. Urol, 2003; 170: 2412–2416. doi: 10.1097/01.ju.0000097383.09743.f9.
- Wu D, Shen N, Lin X, Chen X. Prepubertal testicular tumors in China: a 10-year experience with 67 cases. Pediatr Surg Int, 2018; 34: 1339–1343. doi: 10.1007/s00383-018-4366-6.
- Woodward Paula J, Sohaey Roya, O’Donoghue Michael J, Green Douglas E. From the Archives of the AFIP. RadioGraphics, 2002; 22(1): 189–216. doi: 10.1148/radiographics.22.1.g02ja14189
- Chung JM, Lee SD. Overview of Pediatric Testicular Tumors in Korea. Korean J Urol, 2014; 55(12): 789-796.
- Ross JH, Rybicki L, Kay R. Clinical behavior and a contemporary management algorithm for prepubertal testis tumors: a summary of the Prepubertal Testis Tumor Registry. J. Urol, 2002; 168: 1675–1678.
- Williamson Sean R, Delahunt Brett, Magi-Galluzzi Cristina, Algaba Ferran, Egevad Lars, Ulbright Thomas M, et al. The World Health Organization 2016 classification of testicular germ cell tumors: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology, 2016; 70(3): 335–346. doi: 10.1111/his.13102.
- Epifanio M, Baldissera M, Esteban FG, Baldisserotto M. Mature testicular teratoma in children: multifaceted tumors on ultrasound. Urology, 2014; 83: 195–198. doi: 10.1016/j.urology.2013.07.046.
- Shapiro E, Strother D. Pediatric genitourinary rhabdomyosarcoma. J Urol, 1992; 148: 1761–1768. doi: 10.1016/S0022-5347(17)37023-4.
- Zhu Y, Zhu Z, Xiao Y, Zhu Z. Case report: Paratesticular rhabdomyosarcoma. Front Oncol, 2021; 11: 629878. doi:10.3389/fonc.2021.629878.
- Bouchikhi AA, Mellas S, Tazi MF, Lahlaidi K, Kharbach Y, Benhayoune K, et al. Embryonic para testicular rhabdomyosarcoma: A case report. J Med Case Rep, 2013; 7: 93.
- Taskinen S, Fagerholm R, Aronniemi J, et al. Testicular tumors in children and adolescents. J Pediatr Urol, 2008; 4: 134-137. doi: 10.1016/j.jpurol.2007.10.002.
- Kreydin Evgeniy I, Barrisford Glen W, Feldman Adam S, Preston Mark A. Testicular Cancer: What the Radiologist Needs to Know. American Journal of Roentgenology, 2013; 200(6): 1215–1225. doi: 10.2214/AJR.12.10319.
- Heidenreich A, Paffenholz P, Nestler T, et al. Primary and postchemotherapy retroperitoneal lymphadenectomy for testicular cancer. Oncol Res Treat, 2018; 41: 370–378.
- Ory J, Blankstein U, Gonzalez DC, et al. Outcomes of organ-sparing surgery for adult testicular tumors: a systematic review of the literature. BJUI Compass, 2021; 2: 306–321.
- Sangüesa C, Veiga D, Llavador M, et al. Testicular tumors in children: an approach to diagnosis and management with pathologic correlation. Insights Imaging, 2020; 11: 74.
- Chung JM, Lee SD. Overview of pediatric testicular tumors in Korea. Korean J Urol, 2014; 55: 789–796.
- Faure A, Diakité ML, Panait N, Chaumoître K, Rome A, Merrot T. Le rhabdomyosarcome para-testiculaire de l´enfant: une urgence scrotale. Arch Pediatr, 2012; 19(12): 1340–1344.
- Grogg J, Schneider K, Bode PK, Kranzbühler B, Eberli D, Sulser T, et al. Sertoli cell tumors of the testes: systematic literature review and meta-analysis of outcomes in 435 patients. Oncologist, 2020;25(7): 585–590. doi: 10.1634/theoncologist.2019-0692.
- Bois JI, Vagni RL, de Badiola FI, Moldes JM, Losty PD, Lobos PA. Testis-sparing surgery for testicular tumors in children: a 20-year single center experience and systematic review of the literature. Pediatr Surg Int, 2021; 37: 607–616.

