Infratrigonal Vesicovaginal Fistula Post Pelvic Fracture
Seffar A*, Doumer A, Daghdagh Y, Kbirou A, Moataz A, Dakir M, Debbagh A and Abouateib R
Urology Department, CHU Ibn Rochd-Casablanca, Morocco
Received Date: 21/03/2024; Published Date: 08/08/2024
*Corresponding author: Seffar A, Urology Department, CHU Ibn Rochd-Casablanca, Morocco
Abstract
Isolated infratrigonal vesicovaginal fistula without urethral injury occur after pelvic fracture is extremely rare. We discuss a 26-year-old virgin female patient who developed a vesicovaginal fistula as a result of a serious pelvic fracture which required bilateral internal iliac artery ligation. During the initial trauma, genitourinary injury was missed, patient vital and life-threatening risk was put in the first place. It is only afterwards that it will be managed. Our case (isolated vesicovaginal fistula) is unique since most cases reported in the literature involve either solitary urethral trauma or urethral injury combined with a vesicovaginal fistula. In addition, we use iconography in order to efficiently explain the laparoscopic surgical method step by step.
Keyword: Non‐obstetric fistula, vesicovaginal fistula, bilateral internal iliac artery ligation, pelvic fracture, buttock eschar, laparoscopic repair.
Introduction
Pelvic fractures caused by blunt force trauma, in addition to increasing the mortality, put the genito-urinary organs at danger of injury. Research suggests that approximately 6% of individuals experience this form of genitourinary lesions [1].
The increasing incidence of injuries to the urogenital tract associated with sequelae is caused by a growing number of pelvic ring fractures and, more importantly, by reduced mortality in patients with severe trauma to the pelvic ring [2].
Here we report our experience with a case in a female patient who suffered from a severe pelvic trauma which required initially bilateral internal iliac artery ligation and a prolonged intensive care unit stay, then transferred to the plastic surgery department for a buttock eschar as a consequence of the decubitus position but also the internal iliac ligation. It was only after removing the urinary catheters that vaginal leakage appeared and an isolated infra-trigonal Vesicovaginal Fistula (VVF) has been diagnosed.
Case 1
A 26-year-old female presented at the emergency department after a road traffic accident (motorcyclist struck by a car). The patient was confused, and his general condition was rated as critical. Blood pressure was 70/40 mmHg, pulse 150/min and respiratory rate 28/min. Physical examination revealed sensitivity in the pelvic region and deformity and sensitivity in the right ankle and left forearm. X- radiography indicated a displaced fracture of both obturator frames, separation of the symphysis pubis, stable lateral medial right malleolar ankle fracture and isolated right ulna fracture. The patient barely had time for a normal brain scan before she went into hemodynamic shock. She was intubated and taken directly to the operating room. Bilateral internal iliac artery ligation was done in front of a massive retroperitoneal haemorrhage; no bladder injury was noticed. The pelvis, ulna and ankle fractures was manage conservatively. During her stay in the intensive care unit, she had a urinary catheter and did not complain of vaginal leakage. Due to her prolonged hospitalisation in a recumbent position and most probably the internal iliac ligation a left buttock eschar appeared, requiring hospitalisation in the plastic surgery department with a skin graft preceded by a protective colostomy. On discharge and removal of the bladder catheter, the patient noticed a permanent urinary incontinence, wearing three adult diapers a day. After continuity of the bowel was restored, without faecal incontinence, recurrent urinary tract infections (x4/year) caused mainly by Escherichia coli bacteria were noted. She took her a year to mention it. Finally, she came to our outpatient clinic with complaints about persistent vaginal leakage.
A median laparotomy and colostomy scar were observed during the clinical examination, and there was spontaneous urine leakage of unknown urethral or vaginal origin. The vaginal examination revealed intact hymen, a 3/5 anal sphincter tone, preserved territorial perineal sensitivity, and reduced hip mobility. Cystoscopy revealed an infra-trigonal fistula 2 cm above the bladder neck. TheCT- uroscan showed normal size and regular outline of the kidneys, secreting and excreting within the normal timeframe, with no dilatation of the pyelocal cavities. The bladder did not fill, and all the product passed into the vaginal cavity via a 10 mm fistulous path between the bladder trigone and the anterior surface of the vagina, with no detectable urethral fistula (Figure 1).
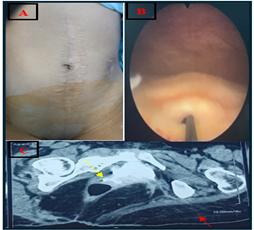
Figure 1: A: median laparotomy and colostomy scar; B: infra-trigonal fistula 2 cm above the bladder neck with a hydrophilic guidewire inserted into vesicovaginal fistula; C: CT cystogram showing excretion of contrast into the vagina (yellow arrow); note the left massive gluteal muscle atrophy.
In this case the choice of approach the vesicovaginal fistula represents a real challenge; firstly, the vaginal approach is difficult to undertake given the restrictive gynaecological position and especially because the patient wanted to keep her virginity. The upper access represents no less of a challenge in this multioperated patient with infratrigonal VVF.
After several appointments detailing the different treatment modalities, their advantages and risks, and the patient's consent, and after obtained a sterile urine culture, a cystoscopy followed by laparoscopic transperitoneal approach has been successfully attempted (Figure 2).
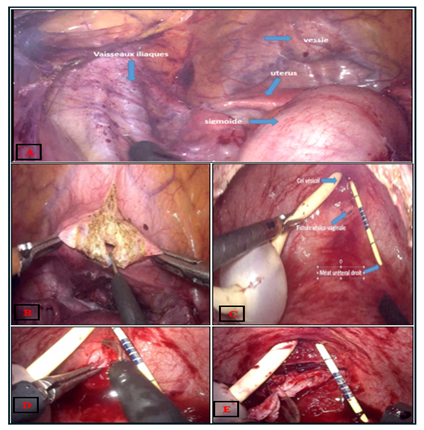
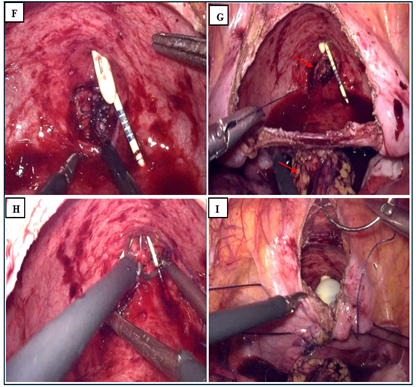
The operation started with a cystoscopy, The fistula was visualized a 16 Fr urethral catheter was inserted to drain the bladder and the ureteral catheter secured to this. The camera trocar was placed superior to the umbilicus and a 30° lens was inserted under direct visualization. The abdominal cavity was examined, and some adhesions were found in relation to his previous surgery. The remainder of the trocars were placed, which included a 5 mm ports at the left and 5 mm at the right rectus margin four fingerbreadths caudal to the camera port, a 10 mm assistant port was placed in the right near the anterior‐superior iliac spine. the table positioned in 30° Trendelenburg. After identifying the iliac vessels, sigmoid, uterus and bladder (Figure 2A), the peritoneum overlying the vesicovaginal space was sharply incised and dissection of the anterior vaginal plane was done without using the vaginal retractor. A wide vertical cystotomy was performed (Figure 2B) and the fistula has been located (Figure 2C). To improve exposure, two sutures were introduced and return through the abdominal wall at the level of the lateral faces of the bladder. (Clearly seen at Figure 2I). Then dissection plane between the vagina and the bladder was developed. The fistula was widely excised obtaining healthy margins of bladder and vagina. (Figure 2D, 2E). The excised tissue was sent to pathology for evaluation. The vaginal defect was closed vertically in a single layer using a running 3‐0 polyglactin (Vicryl) suture (Figure 2F). A well‐vascularized omental flap was brought down to the pelvis to cover the vaginorrhaphy and sutured to the vaginal wall but also to the posterior wall of the bladder with interrupted 3‐0 chromic sutures (red arrows Figure 2G). Then the bladder wall was sutured horizontally in one plan using a running 2‐0 polyglactin (Vicryl) suture (Figure 2H). The cystotomy was closed in one plane using a running 0 polyglactin (Vicryl) suture (Figure 2I).
18 Fr urethral catheter was inserted to drain the bladder and the right/left ureteral catheter secured to this. We checked the integrity of the suture by infusing 250 cc of saline with povidone.
The patient was discharged in postoperative day 5.
A cystography was performed in postoperative day 21 before extraction of the urinary catheter to rule out urine leakage and the ureteral stents were also withdrawn. At 6-month follow-up, the patient remains with the fistula closed and continent. It should be noted that she initially had urgency which were resolved very quickly. Its bladder capacity at control was measured at 310 ml.
Discussion
Fractures of the pelvis are typically the result of high-energy trauma and place patients at risk of associated bladder, urethral, and vaginal injury [3,4].
This Genito-urinary injuries account for 6% in the United States [1] and 2.63% of urogenital fistulas in Africa [5]. The increasing incidence of injuries to the urogenital tract associated with sequelae is caused by a growing number of pelvic ring fractures and, more importantly, by reduced mortality in patients with severe trauma to the pelvic ring [2].
Extraperitoneal rupture of the bladder most commonly occurs when the bladder is lacerated by a sharp, bony spicule. Injury to the bladder or urethra most commonly occurs in association with significant anterior ring disruptions, most commonly pubic ramus fractures [6].
Pelvic fractures often occur by high energy trauma and have a significant mortality rate, with a frequency ranging from 8 to 15% [7,8].
Rothenberger pointed out that 66% of injured people who died of pelvic fractures died of haemorrhage [9]. In our case, a bilateral internal iliac artery ligation was done in front of a massive retroperitoneal haemorrhage. It should be remembered that in our present case, this ligation very probably saved this young patient's life but was complicated by the development of an eschar of the left gluteal muscles.
The principle of hypogastric arteries ligation is based on tying the anterior trunk of the hypogastric artery about 2 cm from the iliac bifurcation in order to preserve the posterior gluteal branches. It can reduce haemorrhage in 40-100% of instances, but it nevertheless has a poor surgical reputation due to the difficulty of the procedure and the associated morbidity [10].
No case of vesico-vaginal fistula occurring in a traumatic setting after ligation of the hypogastric arteries has been reported. We exclude this aetiology, but nevertheless consider it to be an aggravating factor that delays spontaneous closure of vesico-vaginal fistula under bladder catheterisation alone.
When haematuria and vaginal bleeding occur after pelvic fracture, the diagnosis of genitourinary trauma is clinically suspected; however, because the patient's vitals and life-threatening risks were prioritized, their genitourinary injuries were frequently ignored in the emergency room during the primary trauma. Reports have noted that initial emergency department assessment can miss this diagnosis in as many as 40% of cases [1].
The timing of repair remains a point of contention. Several reports immediate repair is recommended for injured mid-urethra and/or bladder neck, as well as concomitant vaginal laceration, as a ruptured female urethra can induce full obliteration due to tissue inflammation and extensive scarring [11,12].
On the other hand, some reports indicate that stage repair may be the best alternative since the patient's situation is occasionally unstable, and cystostomy construction allows pelvic hematoma reabsorption and tissue inflammation decrease [13,14].
The correct fistula identification is the most crucial step in their management. Outside the emergency context, the workup includes pelvic examination with speculum and cystoscopy. In some cases, a fistula tract might be seen during clinical examination or by cystoscopy, although VVF can be very difficult to diagnose. When imaging modalities are not available, a “double dye test” might be helpful to better understand the fistula location. Preoperative understanding is of paramount importance in order to understand the number of fistulae (“hidden fistula”); their size, location, and distance from the ureteral orifices; as well as possible fistula branching. A CT scan with a cystogram can assist identify the specific location of the fistula. Magnetic resonance imaging (MRI) typically shows wall enhancement in active or healing VVFs. Healing VVFs may also include central granulation tissue. Factors influencing the success rate of VVF repair include size, location, prior fistula repair, clinical experience and skills of the surgeon, peri-fistula fibrosis that depends on the etiology and clinical course of VVF, and the quality of surrounding tissue such as peritoneum or sigmoidal epiploic appendices. Vesicovaginal fistulas can be treated using multiple ways, including conservative therapy, which has an 20% success rate [15]. Nonoperative treatment provides poor results, leading to surgery as the primary treatment option.
Vesicovaginal fistulas can be repaired by vaginal, abdominal, or laparoscopic/robotic techniques. Minimally invasive procedures, such as laparoscopic and robotic approaches, are increasingly used to improve visibility and reduce morbidity in open abdominal repair [16].
Bora et al. reported a 93% success rate in a cohort of 30 patients who underwent robotic assisted VVF repair using omental, sigmoid epiploic, or peritoneal tissue interposition over an average follow-up of 38 weeks [17]. The route depends on both the fistula's characteristics and the surgeon's experience. Traditional VVF repair methods include transabdominal for supratrigonal VVF and transvaginal for low lying fistulae [18]. While supra-trigonal fistulas can be repaired using laparoscopic technique, infratrigonal fistulas typically require transvaginal treatment due to their position [15,19].
Conclusion and Recommendation
In the event of pelvic trauma, vaginal bleeding and haematuria should not be missed. A CT scan with a cystogram must always be done, even in an emergency. Delayed treatment was not fatal in our case. Our case shows the possibility of performing laparoscopic transperitoneal repair of infra-trigonal vesicovaginal fistula with interposed omental flap in this multi-operated young patient, which made it possible to preserve her virginity with probably less morbidity than open surgery with no more urine loss complaints after six-month follow-up.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
- Black PC, Miller EA, Porter JR, Wessells H. Urethral and bladder neck injury associated with pelvic fracture in 25 female patients. J Urol, 2006; 175: 2140–2145.
- Pavelka T, Houcek P, Hora M, Hlavácová J, Linhart M. Urologické poranení pri zlomeninách pánevního kruhu [Urogenital trauma associated with pelvic ring fractures]. Acta Chir Orthop Traumatol Cech, 2010; 77(1): 18-23.
- Velazquez N, Fantus RJ, Fantus RJ, Kingsley S, Bjurlin MA. Blunt trauma pelvic fracture-associated genitourinary and concomitant lower gastrointestinal injury: incidence, morbidity, and mortality. World J Urol, 2020; 38(1): 231-238. doi: 10.1007/s00345-019-02725-7.
- Li P, Zhou D, Fu B, Song W, Dong J. Management and outcome of pelvic fracture associated with vaginal injuries: a retrospective study of 25 cases. BMC Musculoskelet Disord, 2019; 20(1): 466. doi: 10.1186/s12891-019-2839-y.
- Lugagne PM, Leo JP, Richard F. Fistules urogénitales. EMC Gynécologie, 1991; 220-310.
- Taffet R. Management of pelvic fractures with concomitant urologic injuries. Orthop Clin North Am, 1997; 28(3): 389-396. doi: 10.1016/s0030-5898(05)70296-0.
- Balogh Z, King KL, Mackay P, McDougall D, Mackenzie S, Evans JA, et al. The epidemiology of pelvic ring fractures: a population-based study. J Trauma, 2007; 63(5): 1066–1073. doi: 10.1097/TA.0b013e3181589fa4.
- Hauschild O, Strohm PC, Culemann U, Pohlemann T, Suedkamp NP, Koestler W, et al. Mortality in patients with pelvic fractures: results from the German pelvic injury register. J Trauma, 2008; 64(2): 449–455. doi: 10.1097/TA.0b013e31815982b1.
- Rothenberger DA, Fischer RP, Perry JF. Major vascular injuries secondary to pelvic fractures. Am. J. Surg, 1978; 136: 660-662.
- Sergent F, Resch B, et al. Intractable postpartum haemorrhages: Where is the place of vascular ligations, emergency peripartum hysterectomy or arterial embolization? Gynecol Obstet Fertil, 2004; 32(4): 320–329.
- Bredael JJ, Kramer SA, Cleeve LK, Webster GD. Traumatic rupture of the female urethra. J Urol, 1979; 122: 560–561.
- Perry MO, Husmann DA. Urethral injuries in female subjects following pelvic fractures. J Urol, 1992; 147: 139–143.
- Hemal AK, Dorairajan LN, Gupta NP. Posttraumatic complete and partial loss of urethra with pelvic fracture in girls. An appraisal of management. J Urol, 2000; 163: 282–287.
- Podesta ML, Jordan GH. Pelvic fracture urethral injuries in girls. J Urol, 2001; 165: 1660–1665.
- Marco Randazzo, Linda Lengauer, Charles-Henry Rochat, Achilles Ploumidis, Darko Kröpfl, Jens Rassweiler, et al. Best Practices in Robotic-assisted Repair of Vesicovaginal Fistula: A Consensus Report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology, European Urology, 2020; 78(3).
- Sotelo R, Mariano MB, Garcia-Segui A, Dubois R, Spaliviero M, Keklikian W, et al. Laparoscopic repair of vesicovaginal fistula. J Urol, 2005; 173(5): 1615–1618.
- Bora GS, Singh S, Mavuduru RS, Devana SK, Kumar S, Mete UK, et al. Robot assisted vesicovaginal fistula repair: a safe and feasible technique. Int Urogynecol J, 2016.
- Tenggardjaja CF, Goldman HB. “Advances in minimally invasive repair of vesicovaginal fistulas,” Current Urology Reports, 2013; 14(3): pp. 253–261.
- Manzano JP, Crochik FDS, Pugliesi FG, de Almeida RVS, Melo PAS, Nunes RLV. Robot-Assisted Infratrigonal Vesicovaginal Fistula Repair. Case Rep Urol, 2019; 2019: 2845237. doi: 10.1155/2019/2845237.

