Seronegative Antiphospholipid Syndrome presenting with Right Atrial Thrombotic Mass
Mohammad Nasser Khattab1,*, Mohammad Marwan Alhalabi2, Ezzat Alousta1, Ammar Akid3 and Mhd Yassin Bani Marjeh4
1Department of Cardiology, Al Bassel Heart Institute, Syria
2Department of Cardiology, University of Damascus Faculty of Medicine, Syria
3Head of Coronary Care Unit, Al Bassel Heart Institute, Syria
4Head of Department of Cardiology, Al Bassel Heart Institute, Syria
Received Date: 15/03/2024; Published Date: 07/08/2024
*Corresponding author: Mohammad Nasser Khattab, Department of Cardiology, Al Bassel Heart Institute Dummar Housing Area, 9th Isle, Damascus, Syria.
ORCID ID: 0009-0006-1211-6889
Abstract
Antiphospholipid Syndrome (APLS) is considered an important systemic autoimmune disease owing to its systemic effects that may be life-threatening, especially because of its hypercoagulability manifestations of vein and artery thrombosis and formation of intracardiac thrombus in rare cases.
We report the case of a 39-year-old woman with a previous history of deep vein thrombosis and a right-sided cardiac and inferior vena cava thrombotic mass with small emboli within the pulmonary arterioles. After clinical examination, laboratory tests, and cardiac investigations, a diagnosis of seronegative APLS was made.
Thrombosis within the cardiac cavity can occur because of many systemic or autoimmune disorders. It is considered a life-threatening emergency condition that requires urgent treatment and emergent intervention to prevent systemic and pulmonary embolic complications.
Keywords: Intracardiac Thrombosis; Right Atrial Mass; Seronegative Antiphospholipid Syndrome; Cardiac Imaging Techniques
List of Abbreviations: APLS: Antiphospholipid Syndrome; TEE: Transesophageal Echocardiography; MSCT: Multi-slice computed tomography; DOACs: Direct Oral Anticoagulants
Introduction
Antiphospholipid Syndrome (APLS) is considered an important systemic autoimmune disease due to its systemic effects that may be life-threatening, especially because of its thrombotic manifestations of vein and artery thrombosis and formation of intracardiac thrombus in rare cases [1,2]. In this paper, we report the case of a 39-year-old woman with a history of deep vein thrombosis and a right-sided cardiac and inferior vena cava thrombotic mass with small emboli within the pulmonary arterioles. After clinical examination, laboratory tests, and investigations, a diagnosis of seronegative APLS was made.
Case Presentation
A 39-year-old woman with a history of smoking hookah daily for one year and does not drink alcohol, had a history of Deep Venous Thrombosis (DVT) in the left lower leg for 13 years, which started during the first trimester of her first pregnancy. She was treated with low molecular weight heparin, and was diagnosed with a Covid-19 infection 2 years ago without the need for hospital admission. She had no history of miscarriage, diabetes, or hypertension. The patient came to the emergency department at our hospital suffering from a 6-month history of fatigue, and dyspnea (NYHA: class II-III) accompanied by severe compressing chest pain behind the sternum which spreads to the left arm and between the shoulder blades, which lasted for 30 minutes, and went away gradually. During the last two months, she complained of two episodes of syncope occurring for a period of 2-3 minutes, preceded by palpitations and blurred vision. Physical examination showed a fixed split in the first heart sound S1, which was best heard at the tricuspid valve area. A 12-lead electrocardiogram showed sinus rhythm and a normal axis, without abnormal electrical findings. Chest radiography revealed a normal-sized heart with no abnormalities noted in the heart and lungs. Emergency causes of chest pain and dyspnea were excluded using ECG, laboratory blood tests, and an emergency echocardiogram. However, the presence of a large mass in the right cavity of the heart was disocvered. The patient was then admitted to the CCU for follow-up and continued cardiac investigation to determine and treat the causative disease. The patient was treated with aspirin 81 mg and unfractionated heparin (UFH) (with APTT ranging between 70-90 sec) while bridging to warfarin with an INR that ranged between (2.5-3).
Transesophageal Echocardiography (TEE) was performed on the third day of admission to the CCU. A TEE showed a hyperechoic lobulated mass starting from the middle of the inferior vena cava and extending to the right atrium, prolapsing through the tricuspid valve towards the right ventricle. The mass was also mobile and measured 5.13×1.53 cm. All cardiac chambers were normal in shape and size, had good motility in all cardiac walls, and had good systolic function (Figure 1). Doppler echography of the lower limbs showed normal findings, with no signs of deep vein thrombosis or arterial thrombosis.
The routine blood testing revealed: WBC:6600u/l, HGB:9.8 g/dl, PLT:215000u/l, creatinine:1 mg/dl, urea:26 mg/dl, INR:1.1, PT:13s, ALT:39u/l, AST:42u/l, TSH:1.4 mIU/ml, CRP:2.2 mg/dl, cholesterol:205 mg/dl, TG:230 mg/dl. Moreover, Wright test, Widal test, HBV and HCV tested negative.
MSCT was performed on the third day to rule out the presence of a pulmonary embolism and showed embolic filling defects in the branches of the left pulmonary arterioles. The pulmonary trunk and the left and right pulmonary arteries were normal. Thrombotic filling defects were observed in the inferior vena cava pathway (Figure 2). Cardiac magnetic resonance imaging was done on the sixth day of admission to determine the likely histological structure of the mass, and showed the presence of a mobile thrombotic mass measuring 20 × 35 mm hanging from the atrium towards the ventricle through the tricuspid valve, consistent with an embolus (post-thrombosis of the inferior vena cava). The pulmonary trunk and parenchyma were normal (Figure 3). Cardiac coronary angiography results were also normal (Figure 4).
Laboratory immunological tests were negative for homocysteine, protein S and C activities, factor V Leiden, antithrombin III deficiency, and normal fibrinogen. Antiphospholipid IgG antibodies, Antiphospholipid IgM antibodies, Anticardiolipin IgG antibodies, Anticardiolipin IgM antibodies, lupus anticoagulant, and Anti DNA (ds) also tested negative. In contrast, ANA-HEP-2 tested positive. Thus, the patient was diagnosed with seronegative APLS based on the highly suggestive clinical manifestations. Surgical intervention was discussed with the cardiac surgery team after confirming the diagnosis with the rheumatology team, but the patient refused surgical intervention despite being informed of the seriousness of the condition. We decided to complete the treatment with warfarin, and the patient remained in the CCU for three weeks, during which time the symptoms completely resolved. The mass diameters started to decrease after the fourth week of treatment, and diminished to about 2.0x2.6 cm. The patient was discharged on lifelong warfarin during home treatment with continuous follow-up.
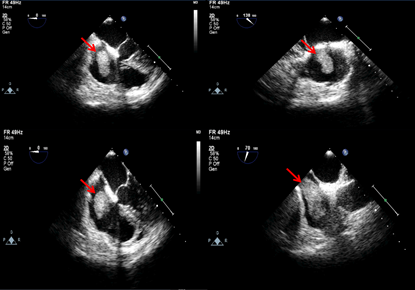
Figure 1: TEE: right atrial mobile mass extended to the right ventricle during diastole.
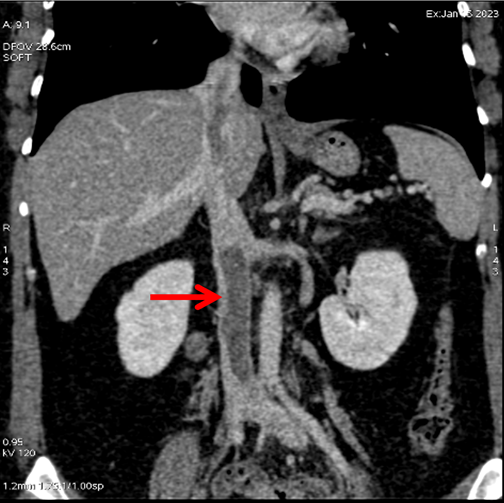
Figure 2: MSCT showed thrombosis in the inferior vena cava.
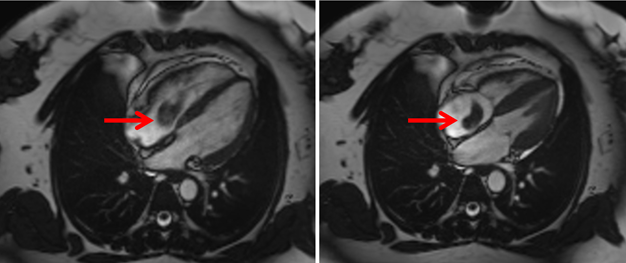
Figure 3: CMR: mobile atrial masse extended to the right ventricular across the tricuspid valve.
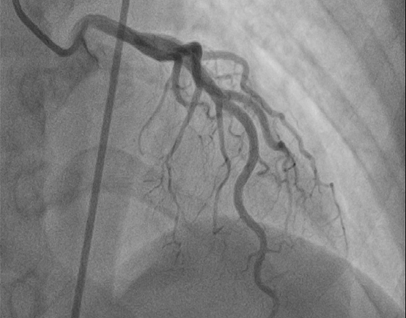
Figure 4: Normal coronary angiography.
Discussion
APLS is a systematic autoimmune disease classified into primary, secondary, and seronegative APLS. The clinical manifestations of APLS are diverse, such as recurrent miscarriages and venous thromboembolism. APL antibodies include Anticardiolipin, Lupus anticoagulant, and anti-beta 2 glycoprotein-I. However, thrombotic events are the most important and life-threatening, as they cause thrombosis within the arteries and veins, and rarely within the heart cavities. The right atrium is reported as the most common site of intracardiac thrombus formation in APLS [2, 3]. In a cohort study of 1000 patients affected by primary and secondary APLS, cardiac involvement was observed in 27% of cases [8], mostly characterized by valvular and coronary artery disease due to accelerated atherosclerosis or thrombosis in the coronary artery. However, cardiac involvement can occur in normal hearts even in the absence of valvular or cardiac dysfunction [4]. Previous studies showed that in rare cases, patients might present with typical symptoms of APLS, such as recurrent thrombotic events, miscarriages, or unexplained thrombocytopenia, but with a transiently positive or a negative test of antiphospholipid antibodies. For these patients, the diagnosis of seronegative APLS has been proposed [5-7]. For the treatment of APLS, oral anticoagulation therapy with warfarin is preferred over direct oral anticoagulants (DOACs). Evidence suggests that warfarin is more effective than DOACs in preventing recurrence of thrombosis and thromboembolism, especially in patients with a history of recurrent thrombotic events [8]. An alternative therapeutic recommendation suggests starting treatment with low-dose aspirin in combination with warfarin (with a target INR of 2.0-3.0). Generally, antithrombotic treatment should be continued lifelong in all patients with APLS [1].
Conclusion
Thrombosis within the cardiac cavity can occur because of a plethora of systemic or autoimmune disorders. Regardless of the cause, it is considered a life-threatening emergency condition that requires urgent treatment and emergent intervention to prevent systemic and pulmonary embolic complications that could end with disastrous and fatal consequences. and Treatment with lifelong oral anticoagulants to prevent the recurrence of such a life-threatening condition is often warranted.
Acknowledgments: We would like to express our gratitude to all doctors at Al Bassel Heart Institute for their support. We also thank the patient and her family for their collaboration.
Authors' contributions: MNK, AJ, MD, and AA approached and followed the patient, and MNK, YBM, and MMA drafted the initial manuscript. All authors revised the final version of the manuscript and approved it for publication.
Conflict of Interest: The authors declare no competing interests regarding the publication of this article.
Funding: None
Ethics approval and consent to participate: Ethical approval for this study was obtained from Al Bassel Heart Institute Board of Directors. Informed consent was obtained from the patient for the publication of this case report.
Consent for publication: Written informed consent was obtained from the patient for the publication of this case report and any accompanying investigations. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Kolitz T, Shiber S, Sharabi I, Winder A, Zandman-Goddard G. Cardiac manifestations of antiphospholipid syndrome with focus on its primary form. Frontiers in immunology, 2019; 10: 941.
- Dhibar DP, Sahu KK, Varma SC, et al. Intra-cardiac thrombus in antiphospholipid antibody syndrome: an unusual cause of fever of unknown origin with review of literature. Journal of Cardiology Cases, 2016; 14(5): 153-156.
- Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nature Reviews Rheumatology, 2011; 7(6): 330-339.
- Hughes G, Khamashta M. Seronegative antiphospholipid syndrome. Annals of the Rheumatic Diseases, 2003; 62(12): 1127.
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. New England Journal of Medicine, 2018; 378(21): 2010-2021.
- Limper M, De Leeuw K, Lely A, et al. Diagnosing and treating antiphospholipid syndrome: a consensus paper. Neth J Med, 2019; 77(3): 98-108.
- Cervera R, Piette JC, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 2002; 46(4): 1019-1027.
- Ghembaza A, Saadoun D. Management of antiphospholipid syndrome. Biomedicines, 2020; 8(11): 508.

