Striking Erythroid Multinuclearity in a Case of Pediatric Acute Myeloid Leukemia with Myelodysplasia Related Changes – a Case Report with Diagnosis Revisited in the Light of Previous vs Updated Classifications!
Omer Javed1, Hamza Khan1,*, Anila Aali1, Khubaib Ahmad2 and Fatima Meraj1
1Haematology department, Indus Hospital and Health Network, Korangi campus, Pakistan
2Bahria University Health Sciences Campus, PNS Shifa, Pakistan
Received Date: 07/02/2024; Published Date: 20/06/2024
*Corresponding author: Hamza Khan, Haematology department, Indus Hospital and Health Network, Korangi campus, Karachi, Pakistan
Abstract
Background: Acute Myeloid Leukemia (AML) having dysplastic changes is categorized in World Health Organization (WHO) Classification of hematopoietic and lymphoid tumors 2017 as Acute Myeloid Leukemia with Myelodysplasia-Related Changes (AML-MRC). It is a high-risk disease of adult population having poor patient outcomes. It is rarely seen in pediatric population. Labelling such a diagnosis in a pediatric patient is a challenge which is dealt with correlation between clinical presentation, morphological dysplasia and cytogenetic/molecular abnormalities. The new updated classifications of hematolymphoid neoplasms have modified this entity and refined it.
Case presentation: Here we present an interesting case of an 11-year-old male child, who presented to us with fever and lymphadenopathy. Bone marrow biopsy performed revealed >20% myeloblasts and >50% dysplasia in erythroid precursors and megakaryocytes. The most interesting finding of the case was bizarre erythroid dysplasia. The case was concluded as AML-MRC as per WHO Classification 2017. The case is revisited in the light of the new updated classifications and key learning points are discussed in detail with review of literature.
Conclusion: In the light of revised World Health Organization (WHO) Classification 2022 and the new International Consensus Classification (ICC) 2022, this case could be either AML with myelodysplasia related gene mutations or AML with myelodysplasia related cytogenetic abnormalities. The present case highlight spectrum of dysplasia in AMLs and show that such cases could be seen in pediatric population as well.
Keywords: AML with myelodysplasia; Erythroid multi-nuclearity; Erythroid dysplasia; Pediatric AML; AML-MR; AML with gene mutations; AML with MDS related cytogenetics
List of abbreviations: AML: Acute Myeloid Leukemia; AML-MRC: Acute Myeloid Leukemia with Myelodysplasia Related Changes; WHO: World Health Organization; ICC: International Consensus Classification; AML MR: Acute Myeloid Leukemia-Myelodysplasia Related; CDA: Congenital Dyserythropoietic Anemia; HSCT: Hematopoietic Stem Cell Transplant; MDS: Myelodysplastic Syndrome; FISH: Fluorescent in Situ Hybridization; H and E: Hematoxylin and Eosin; IHC: Immunohistochemistry
Background
Acute Myeloid Leukemia (AML) having dysplastic changes is categorized in WHO Classification 2017 as Acute Myeloid Leukemia with Myelodysplasia-Related Changes (AML-MRC). It is more frequently found in older AML patients. It is believed to account for a sizable 24–35% of all AML cases and is rare in pediatric population [1]. AML-MRC has a worse prognosis than non-MRC AML, with lower rates of total survival and full remission [2,6]. Additionally, it frequently responds less well to conventional aggressive induction chemotherapy regimens [4,6].
As per WHO Classification 2017, AML-MRC includes cases with 20% or more blasts in the peripheral blood or bone marrow and any one of the following; (1) morphological dysplasia in 50% or more of the cells in ≥ 2 myeloid lineages and/or (2) an antecedent myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative (MDS/MPN) phase and/or (3) characteristic MDS-related genetic abnormalities. This category does not include AML patients with a history of prior cytotoxic therapy (also known as therapy-related myeloid neoplasms) or instances with a recurring chromosomal abnormality that are part of the "AML with recurrent cytogenetic abnormalities" group [1]. However as per the new International Consensus Classification (ICC) 2022 the entity AML-MRC has been replaced by AML with myelodysplasia related gene mutations and AML with myelodysplasia related cytogenetic abnormalities, while the updated World Health Organization (WHO) Classification 2022 now designates this category as AML-myelodysplasia related (AML-MR). Key changes include removal of morphology alone as a diagnostic criterion and update of defining cytogenetic criteria and gene mutations [8,9].
Here we present a case of a child who presented with fever. FBC (full blood count) showed few circulating blasts after which bone marrow biopsy and flow cytometric analysis was performed which revealed >20% myeloblasts along with >50% dysplasia in erythroid precursors and megakaryocytes, thus the case was concluded as AML-MRC as per defined criteria in WHO Classification 2017. The most unique feature in the case was the picturesque erythroid dysplasia particularly marked multi-nuclearity. In the light of updated classifications, the diagnosis is revisited and discussed in the article.
Case Presentation
An 11-year-old male child presented with complaints of fever and generalized weakness for 7 months. Fever was intermittent, not associated with rigors, chills, cough or diarrhea. General physical examination showed pallor and palpable submandibular lymph nodes, the rest of the examination was unremarkable. He was transfused 2 units PRBCs since the onset of illness. There was no history of any previous transfusion. Past medical, surgical and family history was unremarkable. Birth, nutritional and developmental history was normal. There was no history of any prior therapy/chronic drugs usage. Infective workup was negative for viral markers (Hepatitis/HIV/Dengue), blood culture and Malarial parasite. FBC (full blood count) reported Hemoglobin of 9.5 g/dL, WBC count of 2.4 x 10E9/L and Platelets of 83 x 10E9/L. On differential leucocyte count there were12% blast cells (Figure 1A). Bone marrow biopsy was performed for diagnostic workup. Bone marrow aspirate was particulate and cellular. It showed increased blasts comprising of 33% of the marrow nucleated cell population. It exhibited erythroid hyperplasia with significant dyserythropoiesis in 60% of erythroid precursors. Spectrum of dysplastic features include nuclear irregularity, nuclear-cytoplasmic asynchrony and large to giant forms with eye-catching multinuclearity. Upon a further specific differential count of dysplastic erythroid precursors, 49% had multinuclearity while the remaining 51% had other dysplastic changes. Amongst the ones with multinuclearity, 35% had 5 or more than 5 separate nuclei while the rest 65% had less than 5 nuclei (Figure1: B-F). A 500 cells differential count reported 33% blast cells, myeloid precursors 9%, erythroid precursors 46%, lymphocytes 11% and plasma cells 1%. Myeloid to erythroid ratio was reversed and calculated to be 0.2:1. Cytochemical myeloperoxidase stain was negative in blast cells. Trephine sections showed overall cellularity of around 90-95%, with interstitially increased blast cells (Figure 2A). Dysplastic changes were noted in megakaryocytic lineage (in 52% of them) which included presence of micro-megakaryocytes and hypolobated forms. Fibrosis assessed on reticulin stain was WHO Grade MF-0. Immunohistochemical markersCD34, CD117 and CD61 highlighted increased blast cells and dysplastic megakaryocytes, respectively (Figure 2: B-D). Flow cytometric analysis for immunophenotyping was performed on bone marrow aspirate and revealed a population of blast cells gated via SSC/CD45 plot having positive expression for CD34, CD117, CD13, CD33 and HLA-DR. This population showed negative expression of CD45, MPO, CD19, CD79a, CD3, CD66, CD14, CD61, CD42 and Glycophorin A (see below dot plots).
On the basis of >50% bi-lineage dysplasia, >20% blast cells having myeloid lineage immunophenotype and no prior history of chemotherapy, the diagnosis of Acute Myeloid Leukemia with Myelodysplasia related changes (AML-MRC) was favored as per WHO Classification 2017 [1].
Other disease specific work up included karyotypic analysis which showed normal male chromosomal analysis i.e. 46,XY[20]. FISH panel for AML was performed for inv(16)(p13.1q22); CBFB-MYH11 and t(8:21)(q22;q22.1); RUNX1-RUNX1T1 which were negative.
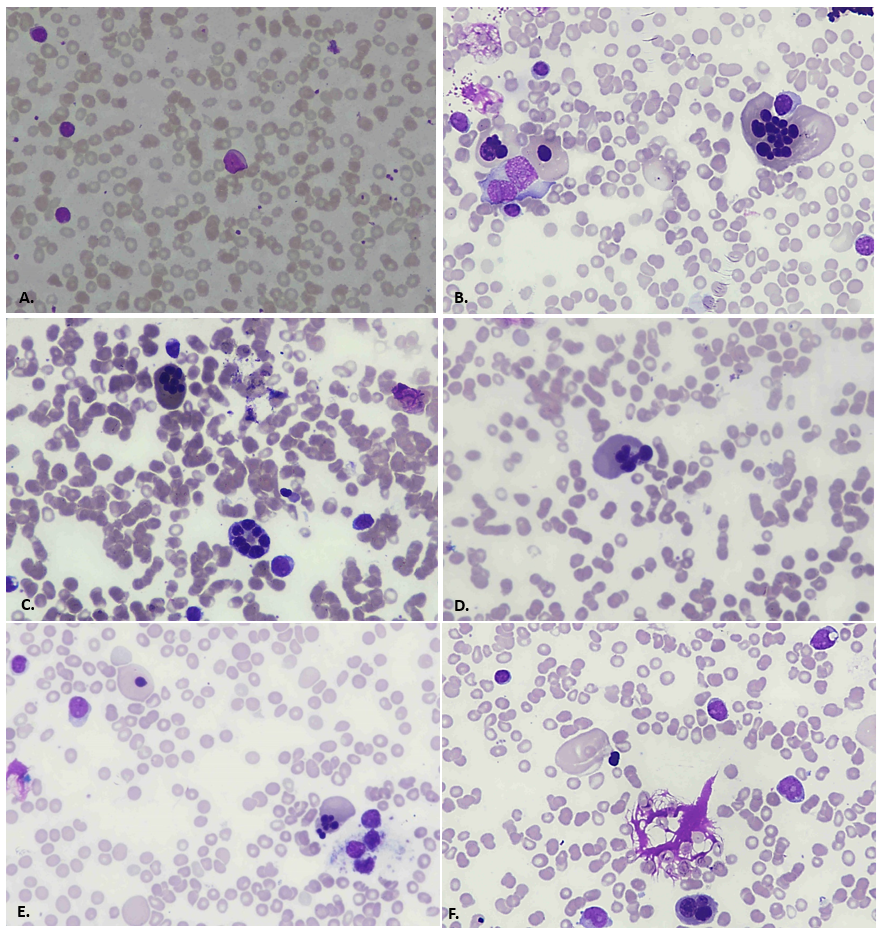
Figure 1: A. 20x objective, Leishman stained; peripheral film showing blast cell in the center. B, C, D, E. 40x objective, Wright-Geimsa stained; bone marrow aspirate showing dysplastic erythroid precursors with prominent multinuclearity. F. 20x objective, Wright-Geimsa stained; bone marrow aspirate showing blast cells and dysplasia in a erythroid precursor.
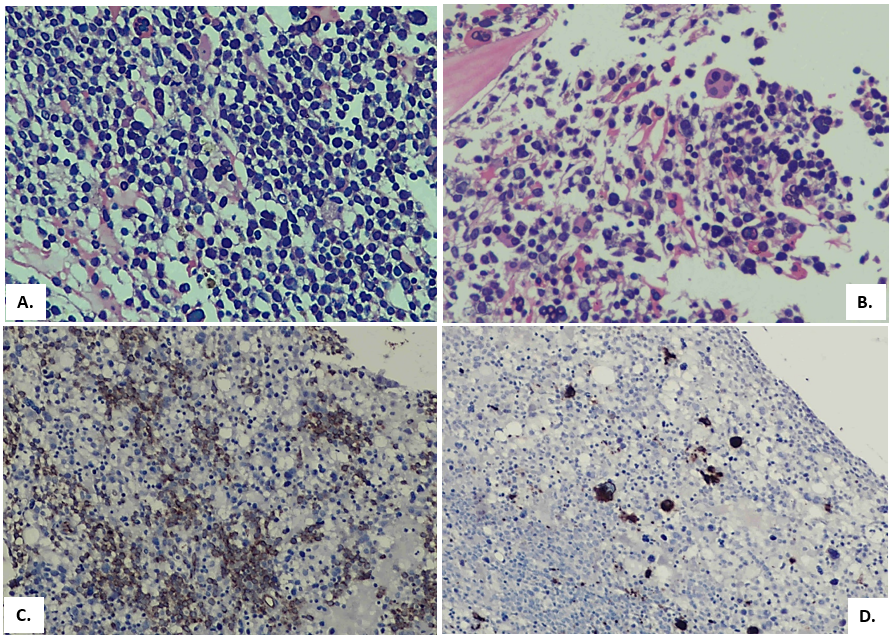
Figure 2: A. H&E stained trephine section, showing hypercellularity with interstitially increased blasts. B. H&E stained trephine section; a dysplastic megakaryocyte is marked. C. IHC marker CD34 highlighting interstitially increased blasts. D. IHC marker CD61 exhibiting micro-megakaryocytes as well as hypolobated forms.
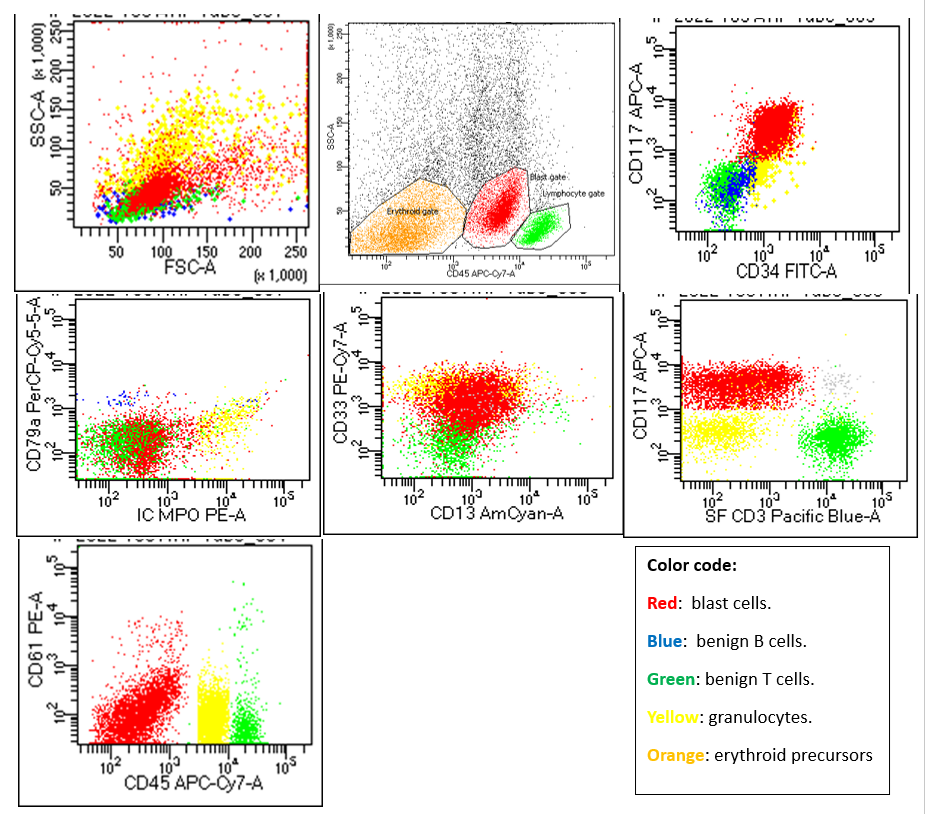
Flow cytometric dot plots: SSC/FSC dot plots show a predominant small to medium sized population in the “blasts” region coded as RED. SSC/CD45 show blasts are dim positive for CD45 as compared to benign T and B cell population. Further dot plots revealed blasts are positive for CD117, CD34, CD33, CD13 and negative for CD3, CD61, CD79a and MPO.
Discussion
A considerable portion of AML cases are acute myeloid leukaemia with myelodysplasia related changes (AML-MRC), which encompass individuals with preceding MDS as well as those with de novo AML who have multilineage dysplasia and/or MDS-related chromosomal abnormalities [1,2]. It is most frequently seen in elderly patients and is uncommon in pediatric population [1,2]. Thus, there is a need for evaluation of MDS related cytogenetic abnormalities, germ-line mutations karyotypic and FISH analysis in suspected cases [4].
The current case presented to us with complaints fever and palpable submandibular lymph nodes on general physical examination. FBC reported pancytopenia after which bone marrow biopsy was performed. Flow cytometry and cytogenetics were also done. Bone marrow examination revealed >20% myeloblasts and >50% dysplasia in erythroid precursors and megakaryocytes, thus as per previous WHO Classification 2017 the case was concluded as AML-MRC. The most interesting finding of the case was bizarre erythroid dysplasia.
Dysplastic features of erythroid lineage can be categorized as either Myelodysplastic syndrome (MDS)-like or Congenital dysrythropoietic anemia (CDA)-like. Commonly seen dyserythropoietic features seen in MDS include prominent nuclear to cytoplasmic asynchrony along with nuclear budding and ringed sideroblasts. While erythroid precursors in CDA exhibit prominent bi- or multi-nucleation as well as inter-nuclear bridging. Our case manifested CDA like dyserythropoiesis including pronounced multi-nuclearity, which is an uncommon finding in a case of AML. The number of nuclei in dysplastic erythroid precursors in our case ranged from 5 to 14, with 35% of multi-nucleated erythroid precursors having more than 5 nuclei while 65% had less than 5 separate nuclei.
The International Consensus Classification (ICC) 2022 as well as World Health Organization (WHO) 2022 updates in AML categories suggest several key differences from prior classifications. In an effort to identify patients with a poorer prognosis than those with AML-NOS, the previous category of AML with Myelodysplasia-Related Changes (AML-MRC) was created [1]. Multilineage dysplasia was identified as a crude proxy for underlying myelodysplasia-related cytogenetic abnormalities, but the association of that condition with a subset of patients who had low-risk gene mutations, particularly NPM1 or biallelic CEBPA, highlighted the need for molecular refinement of that classification [8,9]. The current update refines this entity with genetic and cytogenetic alterations irrespective of the morphologic dysplasia. AML with myelodysplasia-related alterations and therapy-related AML share several characteristics, which further highlights the need for a more precise method of classifying AML [8]. Hence, morphological dysplasia alone is rendered as a weak diagnostic qualifier in such cases.
The new International Consensus Classification (ICC 2022) has replaced the entity AML-MRC and designate it either as AML with myelodysplasia-related gene mutations(defined by mutations in ASXL1, BCOR, EZH2, RUNX1, SF3B1,SRSF2, STAG2, U2AF1, or ZRSR2) or AML with myelodysplasia-related cytogenetic abnormalities (defined by detecting a complex karyotype, del(5q), del(7q), +8, del(12p), i(17q),del(17p), del(20q) and/or idic(X)(q13) clonalabnormalities.8 While the updated World Health Organization (WHO) Classification 2022 now designates AML-MRC as AML myelodysplasia related (AML-MR), described as a neoplasm that develops either spontaneously or after a known history of MDS or MDS/MPN, and that contains ≥20% myeloblasts as well as particular cytogenetic and molecular abnormalities linked to MDS. The diagnosis of AML-MR no longer relies solely on morphology, and the cytogenetic criteria have been updated, among other significant alterations. Mutation-based definition based on a set of 8 genes has been introduced – SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, STAG2, > 95% of which are present specifically in AML arising post MDS or MDS/MPN [9].
Karyotyping of our patient was normal. FISH panel for AML was performed for inv(16)(p13.1q22); CBFB-MYH11 and t(8:21)(q22;q22.1); RUNX1-RUNX1T1 which were negative. Due to non-availability of a referral center and accessible NGS, workup for gene mutation analysis testing couldn’t be performed.
- Our case in the light of updated classification:
The case was revisited in the light of updated classifications. Normal karyotype excludes the entity AML with myelodysplasia-related cytogenetic abnormalities. Germline mutation analysis couldn’t be performed so AML with myelodysplasia-related gene mutations is not yet ruled in or ruled out. Cases with prior history of established Myelodysplastic syndrome (MDS) should be reviewed in the light of the new updated classifications with respect to MDS-related cytogenetic abnormalities or gene mutation analysis as defined in the criteria.
Hemopoietic stem cell transplantation (HSCT) is the preferred medical treatment with a recovery rate of about 60%. The dismal prognosis of AML-MR among children is anticipated to get better with HSCT. In general, it is not advised to give such patients intensive chemotherapy prior to HSCT because it has no survival advantage [6,7].
HSCT was the recommended 1st line treatment option in our case but due to limited number of resources and unaffordability of the patient, he was kept on palliative treatment.
Conclusion
This case highlights the spectrum of dysplastic changes in AMLs. It also shows that AML-MR cases could even be seen in pediatric population. It remains a prevalent and extremely aggressive biologic subtype of acute myeloid leukemia, having unfavorable long-term prognosis. Current research has centered on finding more specialized treatments for patients with AML-MR as well as the genetic processes that cause disease progression in people with antecedent myelodysplasia. Further research aimed at reversing leukemic progression in MDS patients, as well as more efficient and well-tolerated therapies for those who initially present with AML-MRC, are urgently required.
Diagnostic Learning points:
- Is this concomitant AML with Congenital Dyserythropoietic Anemia (CDA)?
This possibility was considered based on the dysplasia seen in erythroid precursors, but due to absence of prior history of anemia and blood transfusions, this is less likely.
- AML with myelodysplasia, in the absence of gene mutations and cytogenetic abnormalities; a puzzle?
In terms of previous WHO Classification 2017, this case is categorized as AML-MRC. However, in the light of revised WHO Classification 2022 and the new ICC 2022 Classification, this case could be either AML with myelodysplasia related gene mutations or AML with myelodysplasia related cytogenetic abnormalities. A normal karyotype is ruling out cytogenetic abnormalities. Testing for associated gene mutations couldn’t be done so this sub-category is a differential as yet.
Ethical approval and consent to participate: Ethical approval not required. Consent taken from the patient’s parents and saved.
Consent for publication: Taken from the patient’s parents and saved
Availability of data and material: Available and saved
Competing interests: None to declare
Funding: None to declare
Authors’ contributions: Dr Omer Javed gave the idea regarding manuscript writing of the case. Dr Anila Aali provided the clinical history and examination findings. Dr Hamza Khan provided the morphological findings with pictures. Khubaib Ahmad searched the literature and extracted relevant references. Dr Fatima Meraj critically evaluated the manuscript. All the authors bear full responsibility of the material provided in the manuscript.
Acknowledgments: None
References
- WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, 2017.
- Arber DA, Erba HP. Diagnosis and Treatment of Patients with Acute Myeloid Leukemia with Myelodysplasia-Related Changes (AML-MRC). Am J Clin Pathol, 2020; 154(6): 731-741. doi:10.1093/ajcp/aqaa107.
- Yabe M, Tang G, Garcia-Manero G, et al. Acute Myeloid Leukemia With t(v;5q33) Is Associated with Poor Overall Survival and Often Lacks Myelodysplastic Features. Clin Lymphoma Myeloma Leuk, 2015; 15 Suppl: S85-S90. doi:10.1016/j.clml.2015.02.007.
- Weinberg O, Pozdnyakova O, Campigotto F, et al. Reproducibility and prognostic significance of morphologic dysplasia in de novo acute myeloid leukemia. Mod Pathol, 2015; 28: 965–976. https://doi.org/10.1038/modpathol.2015.55.
- Weinberg OK, Hasserjian RP, Li B, Pozdnyakova O. Assessment of myeloid and monocytic dysplasia by flow cytometry in de novo AML helps define an AML with myelodysplasia-related changes category. J Clin Pathol, 2017; 70(2): 109-115. doi:10.1136/jclinpath-2016-203863.
- Han TT, Gong XW, Zhang RR, et al. Clinical features and prognosis of childhood acute myeloid leukemia with myelodysplasia-related changes. Zhongguo Dang dai er ke za zhi = Chinese Journal of Contemporary Pediatrics, 2021; 23(3): 271-278. DOI: 10.7499/j.issn.1008-8830.2009176.
- Estey E, Hasserjian RP, Döhner H. Distinguishing AML from MDS: a fixed blast percentage may no longer be optimal. Blood, 2022; 139(3): 323-332. doi: 10.1182/blood.2021011304.
- Daniel A Arber, Attilio Orazi, Robert P Hasserjian, Michael J Borowitz, Katherine R Calvo, Hans-Michael Kvasnicka, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood, 2022; 140(11): 1200–1228. doi: https://doi.org/10.1182/blood.2022015850.
- Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of HaematolymphoidTumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia, 2022; 36: 1703–1719. https://doi.org/10.1038/s41375-022-01613-1.

