Peanuts and Joint Arthroplasty: The Safe Use of Palacos Bone Cement in a Patient Presenting with a Severe Nut Allergy
George Esworthy1,*, Dhanushree Nair2, Akhilesh Pradhan3 and Vikas Sharma4
1MBChB, MSC, United Lincolnshire Hospitals NHS Trust, Lincoln, United Kingdom
2MBBS, United Lincolnshire Hospitals NHS Trust, Lincoln, United Kingdom
3BSc (Hons), MBBS, United Lincolnshire Hospitals NHS Trust, Lincoln, United Kingdom
4MBBS, FRCS (Tr & Orth), United Lincolnshire Hospitals NHS Trust, Lincoln, United Kingdom
Received Date: 02/02/2024; Published Date: 18/06/2024
*Corresponding author: George Esworthy, MBChB, MSC, United Lincolnshire Hospitals NHS Trust, Lincoln, United Kingdom, LN2 5QY
Abstract
Cemented hip arthroplasty is an essential tool in the armamentarium of a lower limb arthroplasty surgeon during the management of patients with severe osteoarthritis. Antibiotic-loaded Palacos cement is widely used and contains refined peanut oil. However, there is poorly defined guidance regarding the safe use of this in patients with severe nut allergies.
We present a case report of a patient undergoing an elective hip replacement where initially an uncemented implant to avoid the risk of an allergic reaction due to the patient’s anaphylaxis to peanuts was planned. Due to intraoperative complications, conversion to a cemented prosthesis was undertaken with successful postoperative results and no adverse complications related to anaphylaxis or allergy.
Incorporating evidence from our case study alongside current literature, we postulate the safe use of bone cement in patients presenting with severe nut allergy. Further studies should assess this relationship in a larger, prospective cohort of patients.
Keywords: Arthroplasty; Peanut Allergy; Bone Cement; Palacos; Hip
Introduction
The use of bone cement has been extremely prevalent within orthopaedic surgery for over 60 years and is a vital material for modern practice. Despite the name cement, the material functions as a ‘grout’ with no adhesive function to create a mechanical lock between implant and bone in arthroplasty surgery [1].
There are many different types available ranging from calcium phosphate to glass polyalkenoate cement [1]. Each has unique properties and can be tailored to certain operative interventions and functions, for example, the viscosity and additivities, e.g., antibiotics. This includes pigmentation for easier visualisation during procedures [2]. Most commonly within the UK, a highly viscous antibiotic-loaded material has been the cement of choice [3].
A common formulation used within total joint arthroplasty is Polymethylmethacrylate (PMMA) based cement. One example is Palacos cement which is primarily formed of a PMMA copolymer (82%); the remaining powder constituents are Zirconium Dioxide, Benzoyl Peroxide, and Gentamicin Sulphate (15%, 1%, and 2% respectively). The liquid component is 98% Methyl Methacrylate and 2% N, N-dimethyl-p-toluidine. Both contain the green colouring Chlorophyll-copper-complex (E141) which uses refined peanut oil as a dilutant [4]. Palacos cement has been shown to have a large market share [5], demonstrating the reliance within practice on this substance and highlighting the importance of understanding its composition.
The decision for cemented versus uncemented hip arthroplasty is dependent upon numerous factors, however, a cemented approach is supported in patients above the age of 70, particularly in women [6].
Despite this, there is limited literature regarding the safe use of bone cement in patients with peanut allergy. However, a recent study has shown that knowledge of peanut oil within bone cement is a relatively unknown fact with only 20% of consultants and 40% of trainees aware of the cement contents [7]. There is anecdotal evidence within our centre that bone cement containing refined peanut oil is safe for use, however, this has not been strongly evidenced within the literature. Our case report presents a patient where bone cement was safely utilised.
Case Report
An 84-year-old lady presented for an elective total hip replacement for progressive osteoarthritic changes requiring surgical intervention. She was noted to have a severe nut allergy with a known previous reaction approximately 10 years ago of anaphylactic symptoms including airway angioedema and haemodynamic instability.
The patient presented with clinical findings consistent of severe osteoarthritis, originating 1.5 years ago with gradual worsening pain and mobilisation over the preceding 2-3 months. She required the assistance of a stick to mobilise and exercise tolerance was limited to 100-200 yards. Upon examination, she presented with an antalgic gait, point tenderness in the groin and limited hip range of motion. Flexion was limited to 70-80⁰ with only 10-15⁰ of internal and external rotation. Her symptoms were isolated to the hip with satisfactory examination findings of the lumbar spine and knee to exclude these as concurrent factors in her presentation.
Severe osteoarthritis was present on the radiographs with anterior acetabular and femoral head osteophytes (Figure 1).
Local guidelines and consensus encourage the use of cemented implants for both acetabular and femoral components for patients above the age of 70 years. Therefore, the patient was listed for a left total hip replacement which was initially planned to be cemented. However, due the severe nut allergy; an uncemented approach was preferred, with concerns regarding the Palacos bone cement containing peanut derivatives.
Standard practice within our unit utilises spinal anaesthesia for hip arthroplasty procedures and therefore the patient was conscious with minimal sedation during surgery.
Preoperative tranexamic acid and antibiotics were administered as per local protocols. A modified Hardinge approach was used with the capsule incised longitudinally and with hip dislocation. The acetabulum was exposed with excision of anterior osteophytes. A 52mm Pinnacle hydroxyapatite-coated cup was implanted with a secure press fit and secured with two screws with a 52/32mm liner satisfactorily placed. During femur rasping, a stable anteromedial calcar split was noted during the final 12mm rasp. Fixation with tensioned steel cable and conversion to cemented implant was considered to ensure stable fixation of the femoral stem. Cementation was therefore a key component of the final implantation strategy. Prior to and during cementation, close discussion with and monitoring by the anaesthetic team was actioned. A consultant anaesthetist was present and regular monitoring of the patient’s observations was performed, including carbon dioxide trace, haemodynamic status and, clinical status. As the patient was conscious, she was evaluated at 2–5-minute intervals, for symptoms like angioedema, vomiting, bronchospasm, and signs of shock during and after the cementation.
Intraoperatively, the patient remained stable with saturations consistently above 97%, heart rate between 55-88 bpm and systolic blood pressure between 100-140. A size 1, 44mm offset, 150mm Exeter long stem was securely implanted. Two mixes of cement were used for stem cementation and pressurisation was performed. A +4/32 mm stainless steel head was implanted. The final position is shown in Figure 2. During cementation and pressurisation, there was no observed change in her haemodynamic status or clinical status.
Whilst in recovery her observations remained stable, and she remained alert with no adverse effects. No antihistamines or other common medications used to treat allergic reaction were required. She progressed well postoperatively with appropriate interaction with the nursing team, with no reports of pain/discomfort, nausea, vomiting, or loss of appetite. Her intra-operative and post-operative observations can be seen in Table 1. She engaged well with physiotherapy and mobilised successfully on the first post-operative day. She was discharged once deemed fit by the physiotherapy and medical teams on the second postoperative day.
She subsequently received follow-up in clinic at the six weeks and was successfully rehabilitated. She mobilised unaided with no complications. Pain was managed with occasional paracetamol and no further opioid analgesia was required upon discharge. She was discharged to a routine follow-up as per the arthroplasty protocols of our unit.
Hence, it is safe to conclude that there were no reports of any adverse allergic reaction during the intraoperative or postoperative period during this patient’s care.
Table 1: Intra and Post Operative Observations.
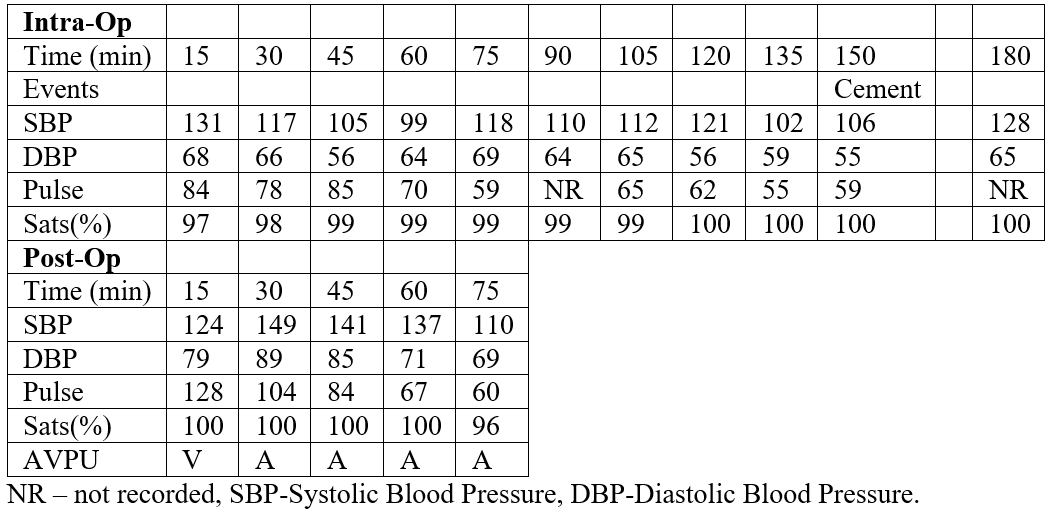
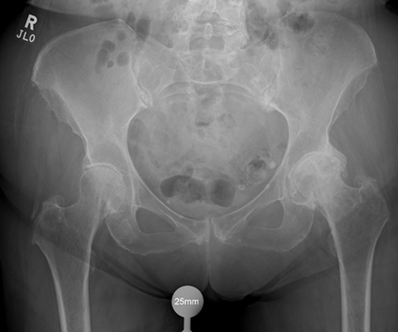
Figure 1: Pre-Operative AP Pelvis radiograph.
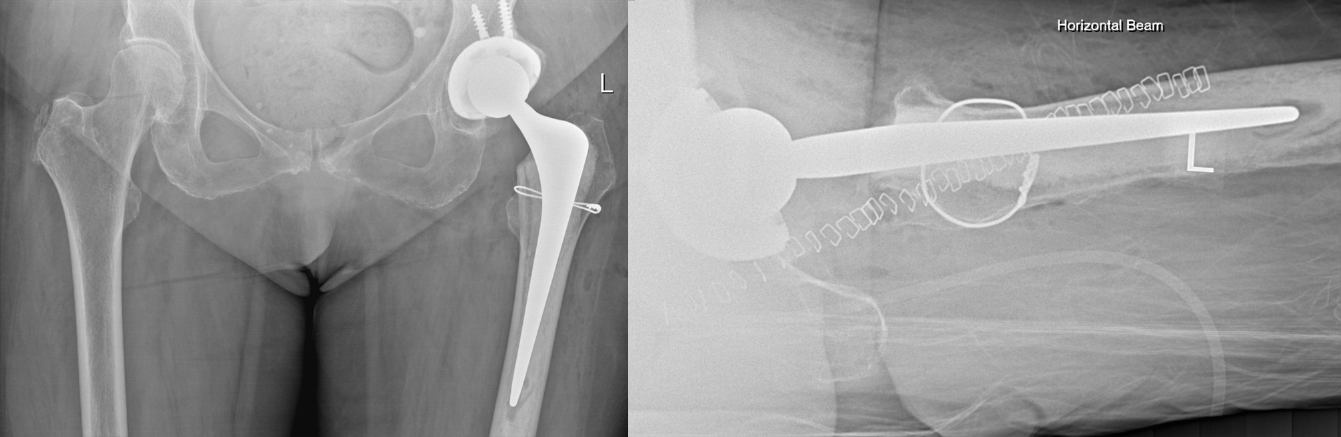
Figure 2: Post-Operative AP Pelvis and Lateral Hip Radiographs.
Discussion
This case illustrates the successful use of refined peanut oil containing PMMA in a patient with a severe nut allergy in concurrence with the conclusion drawn by Ganapathi et al. stating there is no evidence supporting the contra-indication of Palacos cement use in arthroplasty for patients with peanut allergy [7].
This directly contradicts the Heraeus manual for the cement which states that the substance should not be used if a previous hypersensitivity to any of the components is noted [8]. However, this guidance does not inform the reader that any form of peanut oil is present within the cement, instead additional investigation was required to identify that E141 contains peanut oil [2].
Despite this, the peanut oil used is highly refined with laboratory analysis demonstrating no detectable peanut protein with a level below 0.3ng/mL [9]. In practice, this suggests that sensitisation from refined peanut oil is unlikely and therefore a reaction is highly unlikely. A randomised crossover challenge study supports these findings with no peanut allergy patients (n=60) reacting to the refined oil [10]. The Anaphylaxis UK society notes no fatalities related to refined peanut oil, with reported reactions in the minority of individuals being mild [11]. A double-blind cross-over study observed that consumption of peanut oil did not pose risk to individuals with raised serum Ig-E antibodies to both crude peanut extract and purified peanut allergen [12]. Therefore, based on these findings, the use of this bone cement for patients with severe anaphylactic reactions appears safe with no serious reported adverse cases to our knowledge. However, a prolonged period of observation and additional care during initial exposure should be taken.
Given these findings, the consensus for the treatment of patients with nut allergy within joint arthroplasty needs to be addressed. There are currently conflicting views [7] and this case report should provide evidence for a more unified and standardised approach, as these patients can be treated essentially at par with the general population. Furthermore, many patients may benefit from this approach as their primary treatment plan. This is especially important considering the rising prevalence of peanut allergy, as 2% of the Western world is currently affected [13]. Reports of hypersensitivity reactions using bone cement are noted within the literature, although heavy metals are commonly cited as the underlying cause [14].
Interestingly this phenomenon of peanut-derived medical products is not unique to Orthopaedics suggesting a greater awareness of the component aspects of medications is required [15]. This includes the manufacturers producing a comprehensive constituent list to allow for more informed clinical decision-making. Different formulations and further investigations should continue to reduce the allergenicity and potential for sensitisation reactions.
Given the findings reported in the literature and our case report, bone cement products including refined peanut oil and its derivates currently appear safe to use in orthopaedic arthroplasty surgery. Further studies are required to fully address and evaluate the short- and long-term considerations surrounding this.
Conclusion
Palacos bone cement contains peanut oil which is not widely known amongst orthopaedic surgeons. Despite the manufacturer’s guidance, this case report demonstrates the successful use of this product in a patient with severe peanut allergy.
Contributions
Creation of Final Draft: George Esworthy, Dhanushree Nair
Creation of Final Draft and Surgical Case: Akhilesh Pradhan, Vikas Sharma
Two authors were involved in the patient care including surgery.
All authors were actively involved in creation of the case report, including final draft.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Grant Information: No grant is associated with this work.
References
- Vaishya R, Chauhan M, Vaish A. Bone cement. J Clin Orthop Trauma, 2013; 4(4): 157–163.
- Ranjan RK, Kumar M, Kumar R, Ali MF. Bone cement. Int J Orthop Sci, 2017; 3(4): 79–82.
- H12v1NJR, 2023.
- Sandison A, Harrop-Griffiths W. ‘Peanuts and Palacos’. Anaesthesia, 1999; 54(7): 721.
- Bridgens J, Davies S, Tilley L, Norman P, Stockley I. Orthopaedic bone cement. The Journal of Bone and Joint Surgery British volume, 2008; 90-B(5): 643–647.
- Blankstein M, Lentine B, Nelms NJ. The Use of Cement in Hip Arthroplasty: A Contemporary Perspective. J Am Acad Orthop Surg, 2020; 28(14): e586–594.
- Ganapathi M, Jones M, Pumphrey R. Palacos and peanut allergy. Orthopaedic Proceedings, 2005; 87-B(SUPP_I): 51–52.
- PALACOS - bone cements from Heraeus Medical, 2023.
- Peeters KABM, Knulst AC, Rynja FJ, Bruijnzeel-Koomen CAFM, Koppelman SJ. Peanut allergy: sensitization by peanut oil–containing local therapeutics seems unlikely. Journal of Allergy and Clinical Immunology, 2004; 113(5): 1000–1001.
- Hourihane JO, Bedwani SJ, Dean TP, Warner JO. Randomised, double blind, crossover challenge study of allergenicity of peanut oils in subjects allergic to peanuts. BMJ, 1997; 314(7087): 1084–1088.
- Peanut Oil. Anaphylaxis UK, 2023.
- Taylor SL, Busse WW, Sachs MI, Parker JL, Yunginger JW. Peanut oil is not allergenic to peanut-sensitive individuals. Journal of Allergy and Clinical Immunology, 1981; 68(5): 372–375.
- Lieberman JA, Gupta RS, Knibb RC, Haselkorn T, Tilles S, Mack DP, et al. The global burden of illness of peanut allergy: A comprehensive literature review. Allergy, 2021; 76(5): 1367–1384.
- Thomas B, Kulichova D, Wolf R, Summer B, Mahler V, Thomas P. High frequency of contact allergy to implant and bone cement components, in particular gentamicin, in cemented arthroplasty with complications: usefulness of late patch test reading. Contact Dermatitis, 2015; 73(6): 343–349.
- Abed T, Farhat S, Watters G. Naseptin and peanut oil: a survey of practitioners’ awareness in the UK. J Laryngol Otol, 2008; 122(6): 650–652.

