Unveiling the Unknown; A Rare Tale of Rosai-Dorfman Disease’s Intrusion in the Bone Marrow in Pediatric ALL
Omer Javed1, Hamza Khan1,*, Sadia Imran2, Qadarmana Bazai1, Arsalan Bashir3 and Saba Jamal4
1Haematology department, Indus Hospital and Health Network, Pakistan
2Pediatric Oncology department, Indus Hospital and Health Network, Pakistan
3Research Center, Indus Hospital and Health Network, Pakistan
4Blood Center, Indus Hospital and Health Network, Pakistan
Received Date: 31/01/2024; Published Date: 14/06/2024
*Corresponding author: Dr. Hamza Khan, Haematology department, Indus Hospital and Health Network, Karachi, Pakistan
Abstract
Rosai-Dorfman Disease (RDD) is a histiocytic disorder characterized by accumulation of large histiocytes in tissues. The typical presentation is painless lymphadenopathy with B symptoms, or symptoms related to implicated extranodal locations such as the skin, orbit, or central nervous system. Extranodal involvement occurs in approximately 40–45% of cases. RDD has a self-limiting, but long-term clinical course. Bone marrow involvement is extremely infrequent and is associated with poor outcomes. Here we present the case of a 12-year-old male patient, initially diagnosed as B-Lymphoblastic Leukemia, received chemotherapy and achieved remission. Later presented with B symptoms, fits and cytopenias. Upon suspicion of disease relapse, bone marrow biopsy was performed which revealed diffuse infiltration of abnormal large histiocytes. Case was ultimately concluded as bone marrow involvement with RDD.
Introduction
Rosai-Dorfman Disease (RDD) is a histiocytic disorder characterized by accumulation of large histiocytes commonly imparting emperipolesis. It frequently involve cervical lymph nodes. Typical presentation is painless lymphadenopathy with accompanying B symptoms, or symptoms related to involved extra-nodal sites which may include skin, orbit or the central nervous system. Around 40-45% of the cases have extranodal involvement. RDD has generally a self-limited, albeit protracted clinical course. Bone marrow involvement is an extreme rare occurrence and is associated with poorer outcomes. Herein we present case report of a 12-year-old male patient, initially diagnosed with B-Lymphoblastic Leukemia and received relevant treatment. During maintenance cycle of chemotherapy, he developed persistent cytopenias, which were unusual at this phase of chemotherapy. Moreover, he suffered from CNS symptoms including fits. Imaging workup revealed multifocal abnormal signals in lumbosacral region. Due to cytopenias and radiological findings, bone marrow examination was performed which revealed involvement with Rosai-Dorfman Disease. Through this report, we highlight the significance of conducting bone marrow examinations in RDD patients, especially those presenting with cytopenias and atypical symptoms.
Case Presentation
A 12-years-old male patient, presented with complaints of fever and bone pains. Upon further workup, he was diagnosed as B-Lymphoblastic Leukemia and got admitted in Pediatric Oncology unit at Indus Hospital and Health Network, Karachi, Pakistan. He received two cycles of chemotherapy; being stratified as high-risk according to the National Cancer Institute (NCI) criteria. Clinically patient was uneventful during these cycles and his post-induction day 35 MRD (minimal residual disease) was negative. On maintenance cycle 3, he was admitted with complaints of fever, pallor and few episodes of fits. Upon admission, he was given antipyretics, antiepileptic and supportive management.
Hemogram reported cytopenias (Figure 1A). Electromyography (EMG) was consistent with motor axonal neuropathy for which he was treated with painkillers and physiotherapy. Anti-epileptics were also started. MRI spine revealed multifocal abnormal signal intensity lesion in whole of the lumbosacral region suggestive of bone marrow replacement, along with two lesions in L2 and L4 vertebral bodies, suggestive of metastatic deposits. Due to cytopenias and radiological findings, bone marrow biopsy of the patient was performed in suspicion of disease relapse, secondary malignancy or Hemophagocytic Lymphohistiocytosis (HLH).
Bone marrow aspirate was particulate with cellular trails, exhibiting normal trilineage hematopoiesis. Few scattered medium sized atypical histiocytic cells were seen, characterized by single nucleus, open chromatin network and moderate to abundant pale basophilic cytoplasm with occasional vacuolations (Figure 1B). Overt emperpolesis was not seen. Bone marrow trephine showed adequate length core comprising of soft tissue, subcortical and medullary marrow, exhibiting 90-95% overall cellularity. It showed architectural effacement of about 2/3rd of the marrow by diffuse infiltration of medium to large sized histiocytic cells, exhibiting round to elongated bland nuclei and abundant eosinophilic cytoplasm, in a background of marked reticulin fibrosis. Occasional emperipolesis was also present. In the remaining 1/3rd of the core, few inter-trabecular regions of intact marrow architecture with preserved trilineage hematopoiesis were observed (Figure 1C-1H). There was no evidence of Gaucher cells, Neimann-Pick cells, excess blasts, hemoparasite or typical Langerhan’s cells. On immunohistochemistry, these histiocytic cells were positive for CD45, CD163, CD68, S100 and cyclinD1 while negative for CD1a and Langerin (Figure 2(A-G)).
Overall differential diagnoses considered for this bone marrow abnormal histiocytic infiltration included Langerhan’s Cell Histiocytosis (LCH), Erdheim-Chester Disease (ECD) and Rosai-Dorfman Disease (RDD). Morphology was not suggestive of LCH, furthermore negativity of Langerin and CD1a also did not favored LCH. In ECD, touton giant cells are characteristically present, which are S100 negative, in contrast to the findings of our case. So based upon the morphological interpretation, expression of immunohistochemical markers, in correlation with clinical and radiological findings, the case was concluded as histiocytic infiltration in the bone marrow, most likely Rosai-Dorfman Disease.
During the admission, patient developed respiratory distress, along with anemia and thrombocytopenia after which he stepped-up to ICU. His X-ray Chest was consistent with pulmonary infection for which he was being treated with broad spectrum Antibiotics and respiratory support. But there was worsening of respiratory Distress. Intubation was then planned but he went under cardiac arrest and pulmonary hemorrhage. Unfortunately, he couldn’t be revived after Cardiopulmonary Resuscitation (CPR).
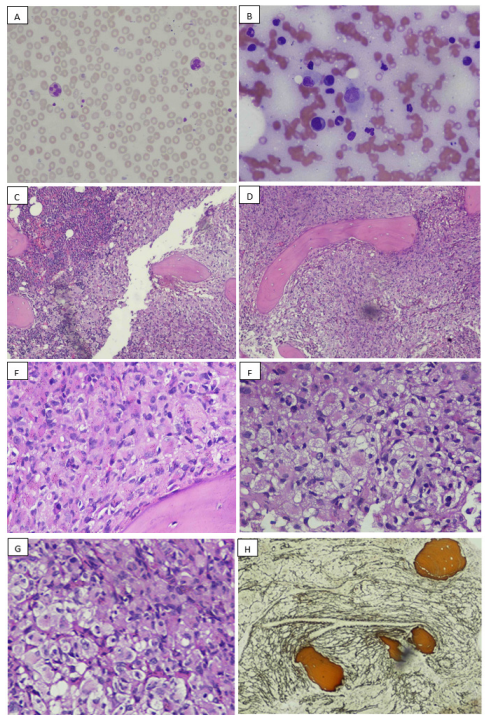
Figure 1: A: peripheral smear, consistent with low blood counts. B: bone marrow aspirate, showing a large sized histiocyte in the center. C: bone marrow trephine, exhibiting replacement of most of the marrow by infiltration of abnormal histiocytes, a focal area of preserved trilineage hematopoiesis can be appreciated at one edge. D: abnormal histiocytic infiltrate, low power. E-G: abnormal histiocytic infiltrate, high power. H: special stain reticulin: exhibiting grade 2 fibrosis.
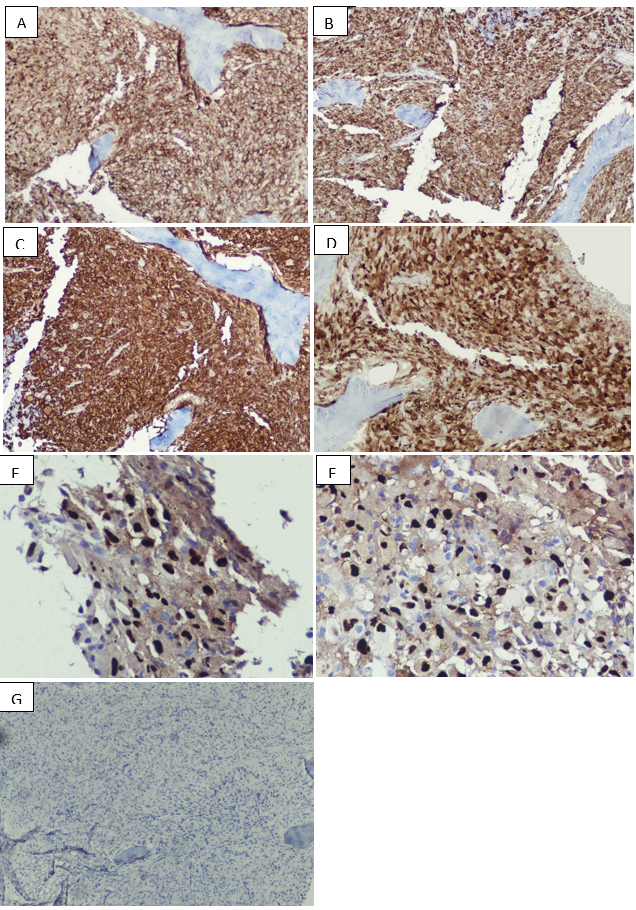
Figure 2: IHC markers: A: CD45 – diffuse positive. B: CD68 – diffuse positive. C: CD163: bright positive. D: S100 – diffuse positive. E-F: cyclinD1 – positive. G: Langerin – negative.
Discussion
Rosai-Dorfman Disease (RDD) is a non-neoplastic proliferation of non-Langerhans cells [1]. It primarily affects children and young adults, presenting usually with large painless, bilateral cervical lymphadenopathy. It may affect extranodal areas, with the skin being the most common site in about 25-40% of these cases [1,11].
Hematological abnormalities in RDD:
RDD can affect the blood cells and cause various hematologic abnormalities, anemia, neutrophilia and thrombocytopenia [7,8].
Hematological abnormalities observed in RDD encompass normocytic normochromic anemia (present in 67% of cases), leukocytosis with neutrophilia (occurring in 60% of cases), and thrombocytopenia [6]. Similar to the case reported by Huang et al., our patient exhibited both anemia and thrombocytopenia, while in the case presented by Zanelli et al., the patient displayed mild pancytopenia [7,10]. Bone involvement is reported in 5% to 10% of RDD cases [6]. Its common manifestation is bone pain with osteolytic bone lesions [5,6]. Although bone marrow infiltration is seldom documented in the literature [6,7,10] the first instance of extranodal RDD involving the bone marrow was reported by Huang et al. in 2006 [7]. Historically, bone marrow exploration is not routinely conducted as a staging procedure in suspected RDD cases, contributing to the scarcity of reported cases involving bone marrow in RDD [7,10]. According to the consensus guidelines for the diagnosis and treatment of RDD established by the American Society of Hematology, individuals experiencing unexplained cytopenias or abnormal peripheral blood cells are advised to undergo bone marrow aspiration and biopsy [6].
The pathophysiology of RDD remains poorly understood. Initially regarded as a reactive and non-neoplastic histiocytic disorder, recent studies have identified mutations in NRAS, KRAS, ARAF, and MAP2K1 in both nodal and extranodal RDD cases. Some studies have suggested a potential association with herpes viruses, cytomegalovirus, Epstein-Barr virus, and HIV, though no definitive causative link has been established [10]. It's worth noting that our patient tested negative for all these viruses. In about 40% of cases, RDD affects other organs or tissues such as skin, nasal cavity, bone, soft tissue, orbital tissue, and central nervous system [4,5]. Our patient had a history of neuropathy and seizure, which could indicate that RDD had spread to the spine and these findings were confirmed on EMG and MRI scan. Bone marrow involvement is extremely rare, with only a few isolated cases described. RDD patients do not typically have bone marrow biopsy performed as staging workup, which may explain the infrequent finding of bone marrow involvement.
The diagnosis of RDD is based on the microscopic examination of tissue sample, which shows replacement of the normal architecture by large histiocytes, with pale bland nuclei and ample cytoplasm [1,3]. These histiocytes have a distinctive feature of engulfing other cells, such as lymphocytes, within their cytoplasm. This phenomenon is called emperipolesis and is a hallmark of RDD [9]. However, emperipolesis is not unique to RDD and is not essential for making the diagnosis. In our patient, we did not find any evidence of emperipolesis in the bone marrow. Abnormal histiocytes of RDD show positive expression of S100 and cyclin D1, apart from the usual histiocytic markers and are negative for the other characteristic LCH-defined markers which include Langerin and CD1a [1,8,12]. This pattern of expression was exactly found in our case.
Abnormal laboratory test results such as hyperferritinemia and high erythrocyte sedimentation rate are not specific and may be related to inflammation or infection [5]. It is recommended to carry out antinuclear antibodies and rheumatoid factor tests in order to screen for an associated immune disease [6]. These tests were negative in our patient, thus, there was no evidence of associated autoimmunity. Many therapeutic strategies exist and depend on RDD manifestations, among these treatments: corticosteroids, chemotherapy, radiotherapy, immunomodulatory therapy and surgery [6,9].
Conclusion
RDD may affect extranodal areas, with the skin being the most common site in about 25-40% of these cases. Primary bone marrow involvement is extremely rare, with only a few isolated cases described. RDD patients do not typically have bone marrow biopsy performed as staging workup, which may explain the infrequent finding of bone marrow involvement. To the best of our knowledge, this is the first reported case of RDD's bone marrow involvement associated with a BALL undergoing maintenance chemotherapy phase with no evidence of primary leukemic disease.
RDD is a rare and benign histiocytic disorder. However, bone marrow involvement in RDD is associated with unfavorable prognosis. Hematologists and pathologists must be mindful of the possible infiltration of bone marrow despite its uncommonness.
According to the American Society of Hematology consensus recommendations for the diagnosis and management of RDD in 2018, it is required for RDD patients with unexplained cytopenias or abnormal peripheral blood cells to undergo bone marrow aspiration and biopsy.
Patient’s Consent: Informed consent taken from the patient’s parents and saved.
References
- World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissues, IARC, 2022.
- Amoako E, Danso KA, Akuaku RS, Ulzen-Appiah K. A Report of Rosai–Dorfman Disease in an Adolescent. Case Reports in Pediatrics, 2022; 2022: 9571400.
- Kumar B, Karki S, Paudyal P. Diagnosis of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) by fine needle aspiration cytology, 2008; 36(10): 691-695.
- Hashimoto K, Kariya S, Onoda T, Ooue T, Yamashita Y, Naka K, et al. Rosai‐dorfman disease with extranodal involvement, 2014; 124(3): 701-704.
- Bruce-Brand C, Schneider JW, Schubert PJJoCP. Rosai-Dorfman disease: an overview, 2020.
- Abla O, Jacobsen E, Picarsic J, Krenova Z, Jaffe R, Emile J-F, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease, 2018; 131(26): 2877-2890.
- Rahali FZ, Taher F, Nassih H, Sayagh S. Bone Marrow Infiltration in Rosai-Dorfman Disease. Case reports in hematology, 2022; 2022: 3420311.
- Zanelli M, Goteri G, Mengoli MC, Capelli D, De Marco L, Valli R, et al. Rosai-Dorfman Disease Involving Bone Marrow in Association with Acute Myeloid Leukemia. Int J Surg Pathol, 2019; 27(4): 396-398. doi: 10.1177/1066896918792617.
- Huang Qin, Chang Karen L, Weiss Lawrence M. Extranodal Rosai-Dorfman Disease Involving the Bone Marrow: A Case Report. The American Journal of Surgical Pathology, 2006; 30(9): 1189-1192. DOI: 10.1097/01.pas.0000209846.52046.62.
- Weng X, Yang Y, Zhang M, Cai C, Sun Y, Wu X, et al. Primary intraosseous Rosai–Dorfman disease: An analysis of clinicopathologic characteristics, molecular genetics, and prognostic features, 2022; 12.
- Gaurav G, Aishwarya R, Jason RY, Mithun VS, Bennani NN, Mrinal MP, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica, 2020; 105(2): 348-357.
- Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood, 2016; 127(22): 2672-2681.

