The Clinical Challenge of Acute Aortic Dissection
Kitihoun Mamadou Chimène*, Soumaré Ahamadou, Coulibaly Fatimatou Zahra, Karimou Bondabou abdoul, Ciss Abdoulai and Lucas Guillaume
Department of Cardiology, Niort Hospital Center, Poitiers University Hospital Center, France
Received Date: 29/01/2024; Published Date: 12/06/2024
*Corresponding author: Kitihoun Mamadou Chimène, Department of Cardiology, Niort Hospital Center, Poitiers University Hospital Center, Niort, France
Abstract
Acute aortic dissection is a medical-surgical emergency whose diagnosis is often made post-mortem; this is because of clinic’s polymorphism. This is why through a case of acute aortic dissection discovered by chance in the emergency room of the Niort hospital, we will try to demonstrate the difficulty in the clinical diagnosis of this pathology.
Keywords: Acute aortic dissection; Medical-surgical emergency; Clinical diagnosis
Introduction
Acute aortic dissection is an uncommon pathology: its exact incidence is not known since approximately one in three dissections is never diagnosed. However, its incidence is currently around 2.9 to 3.5 per 100,000 inhabitants in Western European countries [1]. However, it remains a medical-surgical emergency with a mortality of 33% at 24 hours and 50% at 48 hours if it is not recognized [2].
This mortality is explained by the difficulty and delay in establishing the diagnosis; this, by its clinical presentation which is polymorphic and not very specific, mimicking numerous thoracic and abdominal pathologies, even asymptomatic in 10% of cases [3]. Therefore, the diagnostic strategy of DAA represents a major challenge for the clinician because mortality increases rapidly, particularly during the first hours after the start of the dissection.
This is why through a case in which the diagnosis of aortic dissection could be made in the emergency room of Niort hospital and death avoided, we will show the subtlety represented by the clinical presentation in the diagnosis of this pathology; followed by a discussion where we will show that this difficult clinical presentation is the main reason why the diagnosis of aortic dissection is unfortunately made post-mortem [4,5].
Case Presentation
This is a 69-year-old woman who presented with intermittent transfixing chest pain for three days, increased with deep inspiration, without calming factors and resolving spontaneously. The pain is generalized, difficult to characterize according to her, for which her treating doctor put her on morphine. His medical history boils down to high blood pressure treated with Irbesartan 150mg and Propanolol 40mg; she is an active smoker at a rate of 26 PA. She is not followed for aortic insufficiency. Also, she does not have familial ATCD of Marfan or Loeys-Dietz syndrome. The increase in pain prompted his admission to the emergency room.
On physical examination, the blood pressure was 107/73mmHg in the right arm and 102/70mmHg in the left arm, the pulse was 85/minute, the respiratory rate was 18 cycles per minute, the temperature was 37.3 C° and the saturation at 96% under 2L of O2. Height was 1.60m, weight was 65kgs giving a BMI of 25.4kgs/m2. Cardiac auscultation was regular with a diastolic murmur rated at 3/6, heard at the aortic site. The lungs were clear. There were no signs of heart failure or sensory-motor deficit. The remainder of the clinical examination was unremarkable.
Paraclinical examination found negative troponin at 5ng/ml, hemoglobin at 15g/L, creatinine at 50 umol/L, leukocytes at 11,000 elements/mm3 and D-Dimer at 1700 ng/ml. Hypoxia at 62mmHg on blood gas without lactates. The ECG shows an RRS with a concave inferolateral supra-ST without a mirror (Figure 1). Chest x-ray is normal. ETT reveals mild aortic insufficiency and dilation of the ascending aorta to 44 mm (Figure 2). It was also the aneurysmal aorta and moderate elevation of D-Dimers which made it possible to suspect the AD, leading to the performance of the chest CT angiogram which revealed a Stanford A AD, extended from the right coronary sinus to the right coronary sinus. ostium of the TABC on dilatation of the ascending aorta to 45mm. The patient was then transferred the same day to the Potters University Hospital where she had a successful operation (Figure 3).
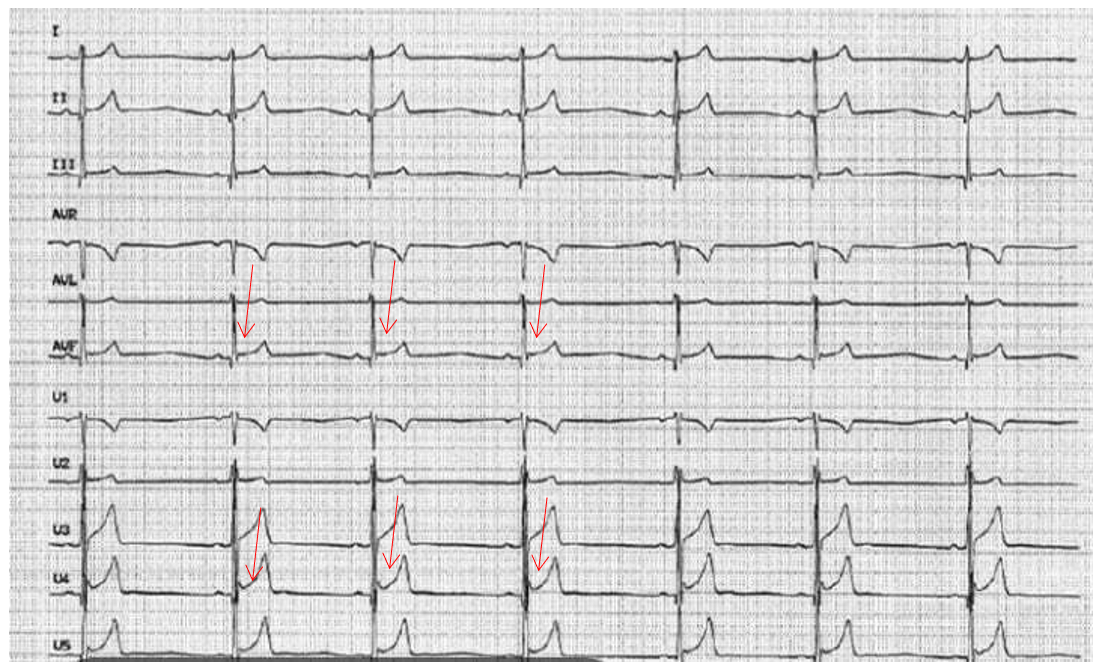
Figure 1: ECG showing early repolarization.
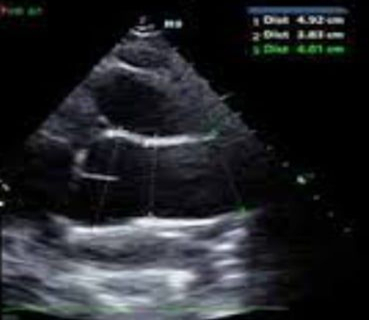
Figure 2: ETT showing dilatation of the ascending aorta.
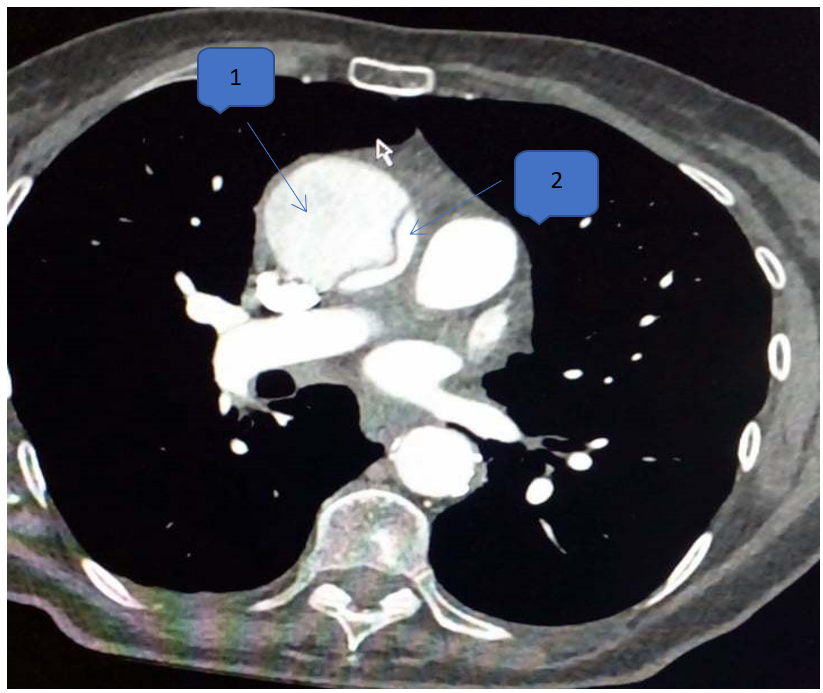
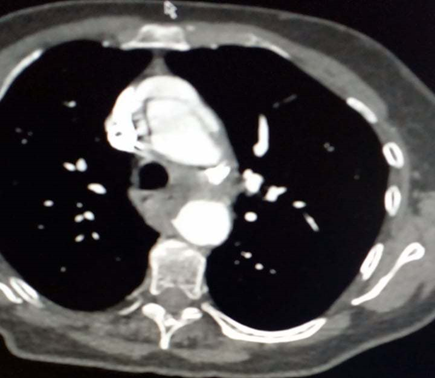
Figure 3: Standard aortic dissection A, extended from the right coronary sinus to the ostium of the TABC on dilation of the AA to 45mm.
1: false channel 2: true channel
Discussion
Chest pain constitutes the main symptom in the clinical presentation of AAD: it is found in 75% of patients [6]. It is characterized by intense, tearing, sudden onset, migratory pain radiating into the back, shoulder blades and lower back. Unfortunately in practice, this classic description of “tearing pain” is found in only 51% of patients, with most describing the pain as piercing or piercing [6]. Furthermore, 5-15% of patients present to the emergency room without any complaints of pain [4-6]. Hypertension is recognized as the main cause of shear stress leading to AD: it is present in nearly 75% of patients with AD [1]. Other risk factors are direct trauma, smoking, hyperlipidemia, cocaine consumption, pregnancy complicated by eclampsia, hereditary diseases of collagen and elastic tissue represented by Marfan syndrome and Loeys syndrome. Dietz in subjects under 40 years of age [1]. Other clinical presentations include aortic insufficiency + aneurysmal aorta (32-45%), loss of pulse or limb ischemia (15-26%), syncope (9-13%), shock or cardiac tamponade (8-18%), hypotension without shock (8-14%), congestive heart failure (6%), focal neurological deficit (5-8%) and finally pericardial rub (2 %) [3,6]. Tension asymmetry is rarely found in practice. AD is predominantly found in males with an average age of 63 years [2,6]. Atypical chest pain in AAD and hypertension as the main risk factor are noted in several literature reviews [1,2,6]: this is also the case for our patient. Furthermore, the polymorphism of the clinical picture mentioned in numerous studies where the diagnosis of AAD was made post-mortem [4,5] is also found in our patient, leading to the suspicion of a pulmonary embolism or pericardial embolism rather than a DAA.
If the clinic is not contributory, the paraclinic is very beneficial in the diagnosis of AD. However, certain tests such as ECG and chest x-ray have poor sensitivity and specificity; notably according to sources from the IRAD (International Registry of Aortic Dissections) and according to the AHA guidelines, a chest x-ray was requested in 100% of patients who finally had a diagnosis of AD, but 10 to 12% were strictly normal and only 60% had the famous enlargement of the mediastinum [3,6]. On ECG, 31% were normal and 41% had non-specific changes in the ST-T segment, 26% had LVH and 15% showed signs of ischemia [3]. The diagnosis is therefore based on 3 imaging methods: CT scan with intravenous contrast, ETT and MRI angiography [7]. However, CT angiography is preferable to MRI angiography given its wide availability, the ability of the majority of radiologists to read it and its reliability now greatly increased with new helical techniques [6]. Furthermore, angiography was once the reference diagnostic test but it has been replaced by the less invasive imaging techniques mentioned above. These findings corroborate those of our patient where the chest X-ray was strictly normal, the ECG was atypical; only ETT and CT angiography made it possible to make the diagnosis and arrive at immediate treatment, thus avoiding death.
Conclusion
Acute aortic dissection remains an uncommon disease with multiple clinical presentations, often insidious, giving it the title of “great imitator”; and with a poor prognosis, meaning that the diagnosis is often made post-mortem. Therefore, it must always be suspected in any chest pain in the same way as acute coronary syndrome and pulmonary embolism.
References
- Jean Marc Alsac. Aortic dissection in the acute phase, The practitioner's review, 2021; 71(8): 842-846.
- Yvan Fournier, Paul-André Moix, Olivier Hugli. Acute aortic dissection: diagnostic utility of D-Dimer.
- Ayrik C, Cece H, Aslan O, et al. Seeing the invisible: Painless aortic dissection in the emergency setting. Emerg Med J, 2006; 23: e24.
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease, JAMA, 2000; 283: pp. 897-903.
- Spittell PC, Spittell JA Jr, Joyce JW, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 trough 1990), Mayo Clin Proc, 1993; 68: pp. 642-651.
- Michel Vallée. A case of aortic dissection in a young adult, Société des sciences vasculares du Québec, 2015.
- Shiga T, Wajima Z, Apfel C, et al. Diagnostic accuracy of transesophageal ultrasound, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med, 2006; s166: 1350-1356.

