Bosniak IV in Native Lumbar Ectopic Kidney: Right Management of Successful Kidney Transplantation
Seffar A1,*, Alafifi M1, Doumer A1, Moatz A2, Dakir M3, Debbagh A3, Cheggali A1, Miara I1, Medkouri G4 and Aboutaieb R5
1Resident in the Department of Urology CHU Ibn Rochd Casablanca, Morocco
2Assistant Professor in the Department of Urology, CHU Ibn Rochd Casablanca
3Professor of higher education in the Department of Urology CHU Ibn Rochd, Casablanca Morocco
4Professor of higher education in the Department of Nephrology CHU Ibn, Rochd Casablanca Morocco
5Head of Urology Department Ibn Rochd University Hospital Casablanca, Morocco
Received Date: 26/01/2024; Published Date: 11/06/2024
*Corresponding author: Seffar A, Resident in the Department of Urology CHU Ibn Rochd Casablanca, Morocco
Abstract
In the event of a suspected renal Bosniak IV cyst on a native right lumbar renal ectopia in a potential kidney recipient, any informed sur-geon is faced with a whole series of challenges, starting with the judicious choice of native nephrectomy technique (laparoscopic or open approach), followed by anatomopathological examination to rule out any signs of malignancy because not all Bosniak category IV cysts are malignant. Finally, the choice of site for the future renal graft must also be made methodically (right or left iliac fossa).
The purpose of this article is to describe, using a clinical case, the standardized procedure that should be followed when a potential recipient candidate is suspected of having a right iliac ectopic renal cyst.
Keywords: Native laparoscopic ectopic nephrectomy; Lumbar ectopic kidney; Renal transplantation; Bosniak 4; Papillary renal adenoma
Introduction
Renal ectopia is a rare congenital abnormality that affects 1 in 3000 people [1]. Such kidneys may be present in a pelvic, iliac, lumbar, abdominal, thoracic, or crossed position. [2]. Renal ectopia can be associated with other renal or extrarenal abnormalities [3]. An elevated risk of cancer does not seem to be associated with ectopic kidneys. It's yet unknown how cancer and ectopic kidneys are related [4,5]. To our knowledge, ectopic kidney on the right side above the level of aortic bifurcation is rare. It seems to us that the laparoscopic method has the benefit of minimally invasive surgery and is appropriate for the treatment of ectopic pelvic kidneys, particularly in potential kidney transplant recipients in such cases. Technical issues with right kidney transplantation can arise, particularly following an open nephrectomy for an ectopic right lumbar kidney.
Case Report
A 24-year-old-man was referred by the renal transplantation service for removal of an asymptomatic, lumbar right kidney before undergoing a planned Living-Related Renal Transplantation (LRRT). The patient had been undergoing haemodialysis for 5 years on a Polycystic Kidney Disease (PKD).
Physical examination revealed a total weight of 86 kg, body mass index 25.8 kg/m2, arterial pressure 120/10 mmHg, a palpable moderately tender discoid shape firm lump (5cm X3cm) in the right lower half of the umbilical and adjoining hypogastric region of the abdomen. The lump was partially mobile. Other regions of the abdomen were soft and non-tender. Bowel sounds were normal. The genital examination and digital examination were unremarkable. Abdominal ultrasound revealed normally positioned left
kidney but the right kidney was not seen in the right renal fossa. Ultra-sounds showed a mass in the right lower quadrant, probably a low right kidney.
Thoraco-abdominal-pelvic angio-scan with 3-dimensional (3-D) reconstructions revealed ectopic right kidney located at L3, small size (6.5 x 4 x 3 cm), polycystic with the most pejorative cyst being medio-renal, multiloculated, with a thick, irregular wall and a thin, calcified septum, with a mural nodule, of 7 mm in diameter, classified Bosniak IV; Two small, permeable right renal arteries, with stenosis at their origin (at L3). The distance between the right inferior renal artery and the iliac bifurcation is measured at 15mm.
A small permeable left renal artery (at L1) and a decreased size left kidney (9 x 4 x 6 cm) with simple cysts (Figure1).
The patient underwent a laparoscopic right nephrectomy via a 4-port approach in the left lateral decubitus position (Figure 2). After the administration of intravenous antibiotics and general endotracheal anaesthesia, a three-way silicone catheter 18Fr was inserted to drain the bladder. The patient was repositioned in the left lateral decubitus position at a 45° angle with the lower leg flexed 90° and the upper leg extended. Access into the peritoneal cavity was done with the open Hasson technique 3 cm above the umbilical. and insufflating with CO2 to a pressure of 15mmHg.The camera (30° lens) was inserted under direct visualization. The abdominal cavity was examined for any sign of injury, adhesions, and identification of anatomic landmarks. The remainder of the trocars were placed as described in Figure 2. The parietal peritoneum was incised along the white line of Toldt of the ascending colon and the colon reflected medially, exposing Gerota’s fascia. The retroperitoneal fascia overlying the renal vessels was carefully separated, exposing the underlying renal veins (two in number). The renal vein was carefully dissected and mobilized. The main renal arteries (two in number) were identified deep to the vein, carefully dissected, mobilized, and divided using the vascular stapling device (Figure 3). Having interrupted the renal vascular inflow, the renal vein was similarly stapled and divided. Gerota’s fascia was mobilized circumferentially, maintaining meticulous haemostasis. The kidney was placed in a specimen retrieval bag and set aside. The insufflation pressure was reduced, confirming the absence of bleeding. The trocars were removed under direct visualization. The periumbilical incision was extended, and the specimen retrieval bag was removed. The specimen was sent to pathology for evaluation. The patient tolerated the procedure well and was taken to the recovery room. Total operative time was 90 minutes, with an estimated blood loss of 50 ml. At the discretion of the renal transplant service, concomitant allograft renal transplantation was deferred secondary to concern over malignancy or infection.
Anatomopathological examination of the surgical specimen revealed the morphological appearance of a 2 cm papillary adenoma associated with numerous simple cysts, as well as chronic interstitial nephritis with calcium oxalate deposits.
No evidence of malignancy was found on the surgical specimen (Figure 4). The patient was not discharged but remained in the renal transplant department for 4 days, and a renal transplant was performed the following day.
The donor was the recipient's 51-year-old mother, who, upon pretransplant workup, was found to have normally placed kidneys. She denied any history of renal calculi, urinary tract infections, or haematuria. She was normotensive, weighed 70 kg (BMI 24.82), and her physical examination was normal. Her serum creatinine was 8.32mg/l, and her glomerular filtration rate (GFR) clearance was 78 ml/min/1.73 m2.
Computed Tomography (CT) angiogram of the donor showed a normally positioned left kidney, right kidney measures 11 cm while the left one measures 10 cm. The renal arteries are unique, the right renal artery measures 17 mm before an early bifurcation into a pre-pyelic and retro-pyelic artery, whereas the left renal artery measures 30 mm before its bifurcation. There was no accessory renal artery. Renal transplantation was classically performed in the right iliac region.
The graft functioned well, and no sessions of haemodialysis were needed after transplantation. Thereafter, the serum creatinine level returned to normal on the 4th post-operative day. Transurethral Foley catheter was removed on the 5th day and the patient was discharged from hospital on day 7.
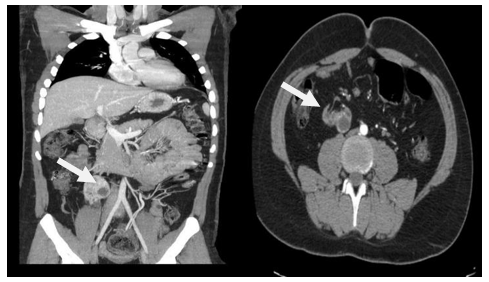
Figure 1A: Abdominal angioscan coronal section in arterial time showing right ectopic lumbar kidney.
B: axial section showing ectopic right kidney with the origin of the renal right artery and Bosniak IV cyst.
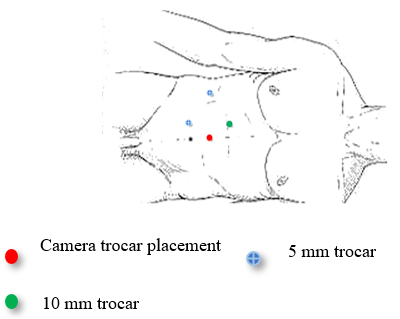
Figure 2: Schema illustrating a laparoscopic right nephrectomy via the 4-port approach in the lateral decubitus position.
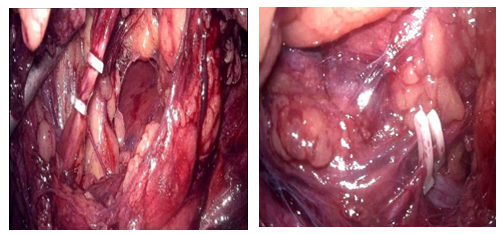
Figure 3: Isolation and division between surgical clips of the anomalous vessels.
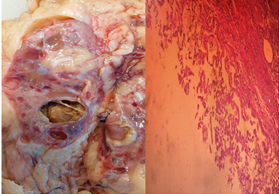
Figure 4: (HE X 40) well-limited cystic lesion surrounding by papillae without atypia or mitosis.
Discussion
Renal ectopy is an uncommon urinary malformation with an incidence of around one in 1000 [6]. It may be high, low or crossed. Low ectopia is most often pelvic but can also be lumbar or iliac. Among these, lumbar kidney is comparatively rare, with a reported incidence of 12% in all ectopic kidneys. [7].
Renal ectopia occurs when the embryonic kidney does not rise normally and can be caused by abnormalities in the metanephros, maldevelopment of the bladder, genetic factors, maternal disease or teratogenic causes. [8].
A predominance of male and left-sided ectopia has been reported in the literature [9]. Most patients with renal ectopy are often asymptomatic, and the ectopy is only detected when there is a complication (hydronephrosis, infection, renal lithiasis, etc.) or when an X-ray or ultrasound is indicated for other reasons.
In the present case report, the ectopic kidney was lumbar on the right side with a renal cyst classified BOSNIAK IV (enhancing 7mm nodule) detected in a preoperative imaging before transplantation. The incidence of renal cell carcinoma RCC in ectopic kidneys is not different from that in normal kidneys. RCC occurring in ectopic kidneys has been previously reported in the literature in approximately 10 cases [10].
According to the EAU guidelines, a Bosniak IV is clearly malignant containing enhancing soft-tissue components. The Managing of Bosniak type IV cysts must be the same as localised RCC (EAU strong recommendation). Surgical and radiological cohorts pooled estimates show a prevalence of malignancy of 0.51 (0.44–0.58) in Bosniak III and 0.89 (0.83–0.92) in Bosniak IV cysts, respectively.
Until the contrary is demonstrated, they are regarded as renal cell carcinomas (RCCs) and onco-surgical management is indicated [11]. Our patient went to a transperitoneal laparoscopic ectopic nephrectomy according to the rules of oncological resection.
In a study by Oueslati et al, 44 patients with cysts classified as Bosniak III in 15 cases (35%) and Bosniak IV in 29 cases (65%) underwent surgical treatment. On anatomopathological examination, 10 (66%) of Bosniak III cysts and 26 (89%) of Bosniak IV cysts were malignant. The benign anatomopathological forms were represented by a tuberculoma, chronic pyelonephritis, a simple serous cyst or xanthogranulomatous pyelonephritis [12]. Also, a foreign body granuloma can mimic a Bosniak IV cyst [13].
The indication of a native laparoscopic radical nephrectomy in our case was first the suspicion of a malignant lesion and helped also to prepare the site of the transplanting kidney due to lack of space for allograft.
The decision to utilize a laparoscopic method was based on two key criteria. First, an angioscannographic examination to highlight arterial and venous abnormalities, preventing maximal surprise per-operatively. As expected, two renal arteries originating directly from the aorta were discovered. Second, the patient was a possible recipient, which made open surgical revision easier, especially since our centre nearly routinely performs a renal transplant in the right iliac fossa.
Surgical procedures can differ from case to case depending on the preoperative and intraoperative picture, and range from radical nephrectomy, nephroureterectomy, laparoscopic radical nephrectomy with or without lymphadenectomy. According to Kumar and colleagues, out of 10 cases of radical nephrectomy for ectopic kidney, only 1 benefited from a laparoscopic approach. This can be explained by the fact that the tumours included in this study were of large mass [14].
In our case, a 2 cm papillary adenoma accompanied with many simple cysts and chronic interstitial nephritis with calcium oxalate deposits were found in the surgical material after anatomopathological evaluation, which goes back to the fact that not all tumours classified as Bosniak IV on a CT scan are malignant.
According to the current WHO classification system, the only histologic distinguishing factor between papillary renal cell carcinoma (PRCC) and papillary adenoma is size, although this size requirement seems somewhat arbitrary. Despite several case reports demonstrating the coincidence of papillary adenoma and PRCC, particularly in the setting of renal adenomatosis [15].
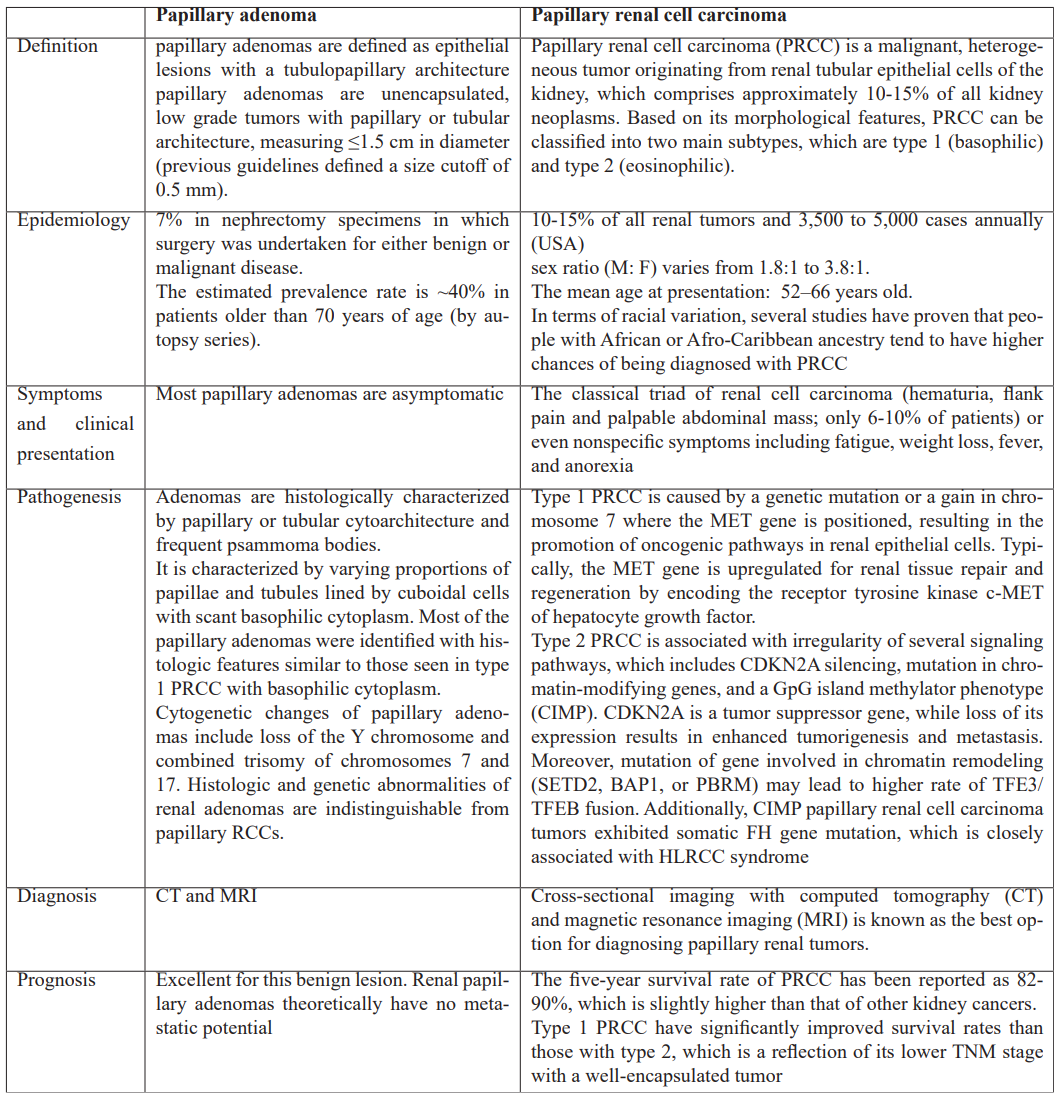
In summary, renal papillary adenoma and PRCC have a high coexistence rate and share histologic and immunohistochemical characteristics, indicating that these two neoplasms may, usually, constitute a continuum of a single biologic process. A possible early step in the formation of papillary neoplasms is increased AMACR expression. There may be a difference in the development of renal adenomas linked to autosomal dominant polycystic kidney disease based on their unique histologic and immunohistochemical profiles [16,17]. The following table summarizes the main characteristics of the two diseases.
Table 1: Characteristics of papillary renal adenoma and papillary renal cell carcinoma [15-17].
Three investigational approaches to the management of autologous kidneys when patients with ADPKD require transplantation have been described in the literature. First, routine (bilateral) nephrectomy can be performed before kidney transplantation to reduce the patient's risk of developing cyst infection after transplantation and therefore the use of immunosuppressive drugs, which can promote and complicate cyst infection Another reason is that if nephrectomy is required after transplantation, there is a risk of kidney graft injury due to surgery-related hypotension or infection [18].
Second, a combined nephrectomy and transplantation procedure can be performed to reduce the number of surgeries. Abrol et al. analysed in 148 ADPKD patients whether a combined laparoscopic bilateral nephrectomy and kidney transplantation is safe compared to kidney transplantation alone. Patients who underwent a combined procedure had longer cold ischemia time, more often a need to be admitted to an intensive care unit, more need for blood transfusions, and a longer duration of hospital stay [19].
Third, a restrictive approach in which nephrectomy is performed only under strict indications, such as severe volume discomfort, lack of space for allograft, recurrent cyst infection, persistent cyst bleeding, or chronic refractory pain. If such symptoms occur, nephrectomy is performed before transplantation. If a patient develops these symptoms after transplantation, a nephrectomy is required. It is estimated in the literature that approximately 40-50% of patients with ADPKD will undergo nephrectomy of one or both kidneys with such a procedure [20].
Successful renal transplantation can reduce cyst size and the prevalence of ACKD, but whether transplantation also reduces the long-term risk of renal cell carcinoma in the native kidney has been controversial. Transplantation can normalize kidney function but increases the risk of cancer due to immunosuppression. The incidence of renal cancer is approximately tenfold in dialysis patients and post-transplant patients compared with the general population [21].
The chronic renal failure and transplantation committee of the French association of urology Transplantation and (CTAFU) recommends after treatment of localized N0M0 CRC (expert opinion):
- no waiting period for tumours of stage stage < pT3 and low-grade ISUP;
- a waiting period of two years for stage pT3 or high-grade ISUP tumours.
- a five-year waiting period for tumours of stage pT4 tumours and rare pejorative histology: medullary carcinoma and translocation carcinoma [22,23].
Conclusion
Right lumbar ectopic kidney with suspicious renal cyst on CT scan grade Bosniak IV is an extremely rare condition, especially in a potential kidney transplant candidate. Fortunately, not all Bosniak category IV cysts are malignant, which as in our case the cyst suspected of malignancy turned out to be a papillary adenoma. Laparoscopic nephrectomy whenever possible, help avoids surgical incision of the future kidney transplant site and also prepare the site of the transplanting kidney due to lack of space for allograft.
References
- Xu YE, Hendahewa R. A rare presentation of an ectopic kidney with pyelonephritis mimicking appendicitis. J Surg Case Rep, 2019; 2019(11): rjz342.
- Sakamoto K, Kojima Y, Takeda R, Terai K, Matsuda M. Solitary pelvic kidney encountered during laparoscopic colectomy. J Minim Access Surg, 2005; 1(3): 133‑135.
- Rodriguez MM. Congenital Anomalies of the Kidney and the Urinary Tract (CAKUT) Fetal Pediatr Pathol, 2014; 33(5‑6): 293‑320.
- Yamamoto T, Watarai Y, Kobayashi T, Matsuda Y, Tsujita M, Hiramitsu T. Kidney volume changes in patients with autosomal dominant polycystic kidney disease after renal transplantation. Transplantation, 2012; 93(8): 794–798.
- Alokour RK, Ghawanmeh HM, Al-Ghazo M, Lafi TY. Renal cell carcinoma in ectopic-pelvic kidney: A rare case with review of literature. Turk J Urol, 2018; 44(5): 433-436. doi: 10.5152/tud.2018.22058.
- Carolan C, Tingle SJ, Thompson ER, Sen G, Wilson CH. Comparing outcomes in right versus left kidney transplantation: A systematic review and meta-analysis. Clin Transplant, 2021; 35(11): e14475. doi: 10.1111/ctr.14475.
- Dretler SP, Olsson C, Pfister RC. The anatomic, radiologic and clinical characteristics of the pelvic kidney: an analysis of 86 cases. J Urol, 1971; 105(5): 623-627. doi: 10.1016/s0022-5347(17)61591-x.
- Gleason PE, Kelalis PP, Husmann DA, Kramer SA. Hydronephrosis in renal ectopia: incidence, etiology and significance. J. Urol, 1994; 151: 1660–1661.
- Higazy A, Shorbagy AA. Surgical management of a locally invasive renal cell carcinoma in an ectopic pelvic kidney, Urology Case Reports, 2020; 29: 101107. https://doi.org/10.1016/j.eucr.2019.101107.
- Atala A, Retik A. Congenital urological anomalies, diseases of the kidney. 6th Edition (vol 1). Ed Schriere RW, Gottschalk CW, Boston. Little Brown and Co, 1997; 1855–1856.
- Hamano I, Hatakeyama S, Soma O, Ishibashi Y, Yoneyama T, Yoneyama T, et al. Renal cell carcinoma in a lumbar ectopic kidney. IJU Case Rep, 2019; 2(3): 124-127. doi: 10.1002/iju5.12056.
- EAU Guidelines. Edn. presented at the EAU Annual Congress Milan, 2023.
- Oueslati M, Saadi A. Profils histologiques et corrélation radio-histologique des kystes rénaux Bosniak 3 et 4 opérés : étude monocentrique, Progrès en Urologie, 2020; 30(13) : 819.
- de Farias LPG, Padilha IG, Dos Santos CJJ, de Miranda CMNR. Not all Bosniak category IV cysts are malignant: foreign body granuloma mimicking renal cell carcinoma. Radiol Bras, 2019; 52(6): 408-409. doi: 10.1590/0100-3984.2017.0197.
- Kumar S, Singh S, Parmar K, Chaudhary K. Multidisciplinary management of a complex case of renal cell carcinoma arising in a pelvic kidney. Annals of the Royal College of Surgeons of England, 2022; 104(3): pages e70 - e73.
- Kim L Wang, David M Weinrach, Chunyan Luan, Misop Han, Fan Lin, Bin T Teh, et al. Renal papillary adenoma-a putative precursor of papillary renal cell carcinoma,Human Pathology, 2007; 38(2): Pages 239-246.
- Lee J, Chae HK, Lee W, Nam W, Lim B, Choi SY, et al. "Comparison of Prognosis in Types 1 and 2 Papillary Renal Cell Carcinoma and Clear Cell Renal Cell Carcinoma in T1 Stage". The Korean Journal of Urological Oncology, 2018; 16(3): 119–125. doi:22465/kjuo.2018.16.3.119.
- Eble JN, Epstein JI, Sesterhenn IA. Pathology and genetics: tumour of the urinary system and male genital organs. Lyon, France; IARC Press, 2004.
- Casteleijn NF, Geertsema P, Koorevaar IW, Inkelaar FDJ, Jansen MR, Lohuis SJ, et al. The Need for Routine Native Nephrectomy in the Workup for Kidney Transplantation in Autosomal Dominant Polycystic Kidney Disease Patients. Urol Int, 2023; 107(2): 148-156. doi: 10.1159/000525575.
- Abrol N, Bentall A, Torres VE, Prieto M. Simultaneous bilateral laparoscopic nephrectomy with kidney transplantation in patients with ESRD due to ADPKD: a single-center experience. Am J Transplant, 2021; 21(4): 1513–1524.
- Yamamoto T, Watarai Y, Kobayashi T, Matsuda Y, Tsujita M, Hiramitsu T. Kidney volume changes in patients with autosomal dominant polycystic kidney disease after renal transplantation. Transplantation, 2012; 93(8): 794–798.
- Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA, 2006; 296: 2823–2831.
- Goujona A, Verhoest G, Sallusto F, Branchereau J, Boutin J-M, Bessede T, et al. Timsit Renal cell carcinoma in candidates for renal transplantation and recipients of a kidney transplant: The French guidelines from CTAFU Progrès en Urologie, 2021; 31(1): pages 18-23.

