Peripartum Respiratory Failure in a Patient with Severe Preeclampsia
Ntioudi Maria1,*, Gelatou Sophia2, Karypidou Victoria3, Alatzidou Dimitra1 and Karagkiouzis Thomas1
1Gynecology-Obstetrics Department, General Hospital of Pella, Hospital Unit of Giannitsa, Greece
2Anesthesiology Department, General Hospital of Pella, Hospital Unit of Giannitsa, Greece
3Intensive Care Unit, General Hospital of Pella, Hospital Unit of Giannitsa, Greece
Received Date: 17/01/2024; Published Date: 31/05/2024
*Corresponding author: Ntioudi Maria, Gynecology-Obstetrics Department, General Hospital of Pella, Hospital Unit of Giannitsa, End of Semertzidi Str, 58100, Giannitsa, Greece
Abstract
Background: Preeclampsia belongs to the spectrum of hypertensive disorders in pregnancy and constitutes a global health issue due to increased risk of maternal, fetal and neonatal adverse outcomes. Acute respiratory failure is an infrequent life-threatening complication in pregnancy caused by either pregnancy disorders including preeclampsia or underlying diseases irrelevant to pregnancy.
Objective: We present a case of a 23-year-old primiparous woman who admitted to our Emergency Department with gestational hypertension in third trimester of pregnancy. The patient was hospitalized in Obstetrics Department for four days for regulation of blood pressure and preeclampsia screening. The patient received antihypertensive treatment promptly. Her laboratory test revealed albuminuria without signs of severe preeclampsia.
Method: On the 4th day of hospitalization, an increased blood pressure was observed despite the antihypertensive treatment, while she complained of severe headaches. It was decided to perform a caesarean section immediately. During the caesarean section, the patient experienced severe bronchospasm that lasted a few minutes with a drop in oxygen saturation. Although there were no auscultatory findings of bronchospasm after 15 minutes, she showed a persistent drop in oxygen saturation and increased needs for oxygen administration. Then, the patient was transferred to the intensive care unit for further monitoring and treatment after undergoing imaging and cardiological assessment. Since major complications ruled out, the patient was admitted to the intensive care unit for a total of 8 days as her oxygen requirements were high.
Results: After a total hospitalization of 14 days, the patient discharged from the Obstetrics Department hemodynamically and respiratoryly stable with an instruction to be reevaluated by a cardiologist and pulmonologist in 15 days.
Conclusion: Severe preeclampsia is the cause of major multisystemic complications that could prove fatal if not recognized and treated appropriately. Acute respiratory failure as a result of severe preeclampsia requires immediate differential diagnosis and treatment.
Keywords: Preeclampsia; Severe preeclampsia; Preeclampsia complications; Respiratory failure
Introduction
Hypertensive disease in pregnancy remains a significant cause of maternal and neonate morbidity and mortality wordwide [1]. Preeclampsia belongs to the spectrum of hypertensive disorders in pregnancy and affects 2% to 8% of pregnant women globally [2]. Additionally, hypertensive disorders constitute the second most prevalent cause of maternal death worldwide, and are responsible for 36–66 % of ICU (Intensive Care Unit) admissions and 10 % of maternal deaths in Europe [3]. Despite improvements in prenatal screening, the administration of aspirin early in pregnancy to prevent placental insufficiency, and improvements in guideline-based monitoring and management of high-risk pregnancies, the fatal complications of hypertensive disorders, although reduced in the developed world, remain a challenge for healthcare providers [4].
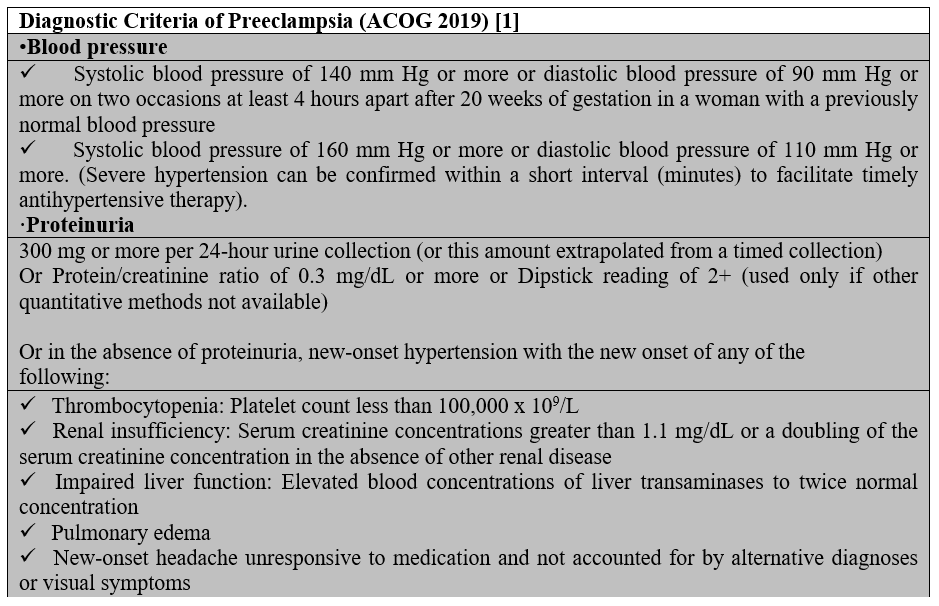
Preeclampsia is associated with new-onset hypertension and proteinuria or other signs and symptoms in the absence of proteinuria [5] (Table 1). Severe preeclampsia occurs in patients with severe range blood pressures (a systolic blood pressure of 160 mm Hg or higher, or diastolic blood pressure of 110 mm Hg or higher) with any of severe features that are described in Table 1.
Pathogenetically, important events in the development of preeclampsia include incomplete trophoblastic invasion of the maternal spiral arteries, poor trophoblastic perfusion and endothelial cell injury with resulting activation of coagulation, impairment of vasodepressor function, altered endothelial permeability and tissue hypoxia [6]. These changes lead to the clinical signs and symptoms of preeclampsia. Hemodynamically, preeclampsia is associated with increased afterload, a normal or low left ventricular preload, a normal or low cardiac output and impaired systolic and diastolic function [7]. As regards the respiratory system, preeclampsia induces an exaggerated capillary permeability which could result in the worsening of the airway edema which may render the intubation very difficult [8]. Furthermore, preeclampsia through the severe tissue hypoxia it causes, leads to an overproduction of lactates, while organic dysfunction prevents their consumption and clearance with a consequent increase in circulating lactates [9]. To conclude preeclampsia appears to be a multi-organ disease frequently with a rapid progress and unpredictable results, caused by endothelial damage, increased endothelial permeability and tissue hypoxia leading to multi-organ dysfunction reacting to the abnormal penetration, development and function of the placenta.
The maternal mortality associated with life-threatening hypertensive disorders (preeclampsia, eclampsia and HELLP syndrome) is principally due to renal failure, coagulopathy (i.e., disseminated intravascular coagulation [DIC]), pulmonary and cerebral edema, placenta abruption, hepatic hemorrhage, and hypovolemic shock [10]. It may present antepartum in 69% of cases with the remaining 31% of cases occurring postpartum. Most of the postpartum cases occur within 48 hours after delivery [11].
Acute respiratory failure requiring mechanical ventilation is a rare complication of pregnancy affecting 0.1 to 0.2 % of pregnancies [12]. More frequently occurs in the postpartum period and is due either to complications of pregnancy such as preeclampsia, amniotic fluid embolism, pulmonary edema, perinatal cardiomyopathy and complications from the administration of tocolytic drugs or to aggravation of underlying diseases not related to pregnancy such as asthma, respiratory infections, heart diseases, pulmonary embolism and gastric acid aspiration [13]. In this report, we present a case of peripartum respiratory failure in a patient with preeclampsia in whom all the above causes were excluded.
Table 1
Case Description
A 23-year-old primiparous woman was admitted to our emergency department due to gestational arterial hypertension at 36 weeks and 3 days of pregnancy. At her admission the blood pressure was 160/100 mmHg, her oxygen saturation 99% and her pulse rate 80 bts/min. The physical examination revealed edemas of lower limbs. The patient was diagnosed with gestational diabetes mellitus at 26 weeks of pregnancy after 2 hour 75-gr Oral Glucose Tolerance Test (0’ 88mg/dl, 60’ 167mg/dl, 120’ 157mg/dl with normal range 0’ < 92mg/dl, 60’ <180mg/dl, 120’ <153mg/dl) well controlled by diet while she was considered to be a high risk pregnancy for the occurrence of preeclampsia already from the first trimester ultrasound examination in combination with the biochemical markers (papp-A at 12 weeks of pregnancy was 0.389 MoM) and therefore she received aspirin 150mg [14] daily until her admission to obstetrics department. Otherwise, the rest of the pregnancy tests were normal. The patient had no medical history, she was fully vaccinated for Covid-19 and she did not smoke. Electrocardiogram of admission was normal. Laboratory studies on her admission showed a white blood cell of 9540/µl (normal range 4-10 K/µl) with 67% neutrophilia (normal range 40-75%), platelet count of 195.000/µl (normal range 150-400 K/µl), glucose 140 mg/dl (normal range 74-106mg/dl), creatinine 0.65mg/dl (normal range 0.51-0.95 mg/dl), SGOT 28 U/l (normal range <35), SGPT 14 U/l (normal range <35), uric acid 6.8 mg/dl (normal range 2.6-6 mg/dl), PCR for COVID-19 was negative and Protein/creatinine ratio of 2.2 mg/dl (normal range <0.3). Once the diagnosis of preeclampsia was made, antihypertensive therapy with methyldopa 500mg 3 times daily, was started immediately. The patient remained in the Obstetric Department for the regulation of blood pressure and monitoring of the well-being of the fetus with the aim of delivery at 37th week of gestation assuming regulated blood pressure to prevent prematurity complications of the fetus. The ultrasound scan of the fetus was normal. Estimated fetal weight was at the 41th percentile while Doppler scanning of fetal vessels was normal [PI UmA (pulsatility index of umpillical artery): 0.92, PI MCA (pulsatility index of mid celebral artery): 2.26 and cpr (cerebroplacental ratio): 2.4]. Additionally, the fetal non stress test (NST) was reactive. After the first 24 hours of administration of antihypertensive treatment, the blood pressure returned to normal levels with an upper value of 135/85 mmHg for 2 days. The 4th day of hospitalization (37 weeks of gestation) patient’s blood pressure was deregulated and despite all antihypertensive treatment it did not drop below 160/110mmHg. Furthermore, the patient complained of severe headaches. Τherefore, a caesarean section is decided due to the fact that bishop score was 1 and because it was considered risky for the patient to experience the stress of normal delivery. Due to the increased risk of peripartum eclampsia, intravenous magnesium was started according to international protocols [1] for the prevention of eclamptic seizures (5gr Mg in 100ml NaCl 0.9% for 20 minutes and then 1-2gr/h iv for 24 hours). The patient was transferred to the operating room and the procedure of introducing regional (spinal) anaesthesia began. Because of failure of nerve block, the anaesthesiologist suggested general anaesthesia. After the patient was intubated and the caesarean section was initiated, she experienced transient bronchospasm (SpO2 80%) which improved immediately (SpO2 94%) after gradual intravenous administration of 5 μg adrenaline (in total 50 μg). Within a quarter of an hour there were no auscultatory findings of bronchospasm. Nevertheless, the patient showed persistent hypoxemia (SpO2 96%) with FiO2 100%. The arterial blood gas (ABG) test revealed pH 7.21 (normal range 7.35-7.45), PO2 106 mmHg (normal range 80-100 mmHg), PCO2 41 mmHg (normal range 35-45 mmHg), HCO3 16.5/ BE -10.7 mmol/L (normal range 22-26 mmol/L), Lactic Acid 3.47 mmol/L (normal value < 2mmol/L). At the end of the operation the patient remained in the operating room awaiting improvement. Despite the administration of fluid intraoperatively (in total 5l of crystalloids), the patient showed reduced diuresis and for that reason it was decided to administer small doses of vasoconstrictors (2-amp phenylephrine 10 mg into 100 ml NaCl 0,9% with a rate of 10 ml/h for two hours) without ceasing the administration of fluid. Two hours later it was decided the patient to be transferred to the Intensive Care intubated and under sedation for specialized monitoring and support. The patient underwent chest X-Ray and a Computed Tomography (CT) of the brain and chest (CTPA, Computed Tomography Pulmonary Angiogram) to rule out serious complications of preeclampsia such as cerebral edema, stroke, pulmonary embolism, and pulmonary edema. Τhe only CT finding was lung atelectasis in the right upper lobe and both lower lobes (Figure 1,2). The CT scan was followed by an echocardiogram to exclude peripartum cardiomyopathy and PFO (Patent Foramen Ovale), other serious complications of preeclampsia that can lead to respiratory failure. Echocardiogram showed good overall left ventricular systolic function without segmental hypokinesias with good wall thickness. EF>50% (Ejection Fraction normal value 50-70%). Good dimensions of left and right heart chambers. Atrioventricular valves were normal.
The patient was hospitalized in the intensive care unit for 8 days. For the first 2 days, she was intubated under sedation with propofol 78μg/min and remifentanil 0.025μg/min. Blood pressure regulation was achieved by intravenous administration of dihydralazine hydrochloride (initial dose 4.92 mg/h and gradual reduction to 1.57 mg/h). Οn the 3th day of hospitalization, the patient showed an improvement in her aerodynamic parameters. Sedation was discontinued and the patient intervened in a T-piece with Spo2 97% for two hours and then she was extubated and due to a drop in SpO2 (89-90%) she was put on NIV (noninvasive ventilation) with PEEP (positive end expiratory pressure) 9mmHg and PS (pressure support) 9 mmHg with FiO2 (fraction of inspired oxygen) 70%. On the 4th day of hospitalization, she was given high-flow oxygen at 60 lt/min with a FiO2 of 50% and on the 5th day she switched to Aquapak System at 8 lt/min with FiO2 at 35% for two days. On 8th day the patient was discharged from the Intensive Care Unit hemodynamically stable with nasal oxygen at 3 lt/min (SpO2 98%) and oral antihypertensive treatment with methyldopa 500 mg 3 times daily. The chest X-Ray was normal (Figure 3). Eventually, the patient remained in the Obstetric Clinic for 2 days and was discharged hemodynamically stable, without the need for nasal oxygen with instructions to continue to receive antihypertensive medication, to monitor blood pressure and follow up with a cardiologist and pulmonologist in 15 days.
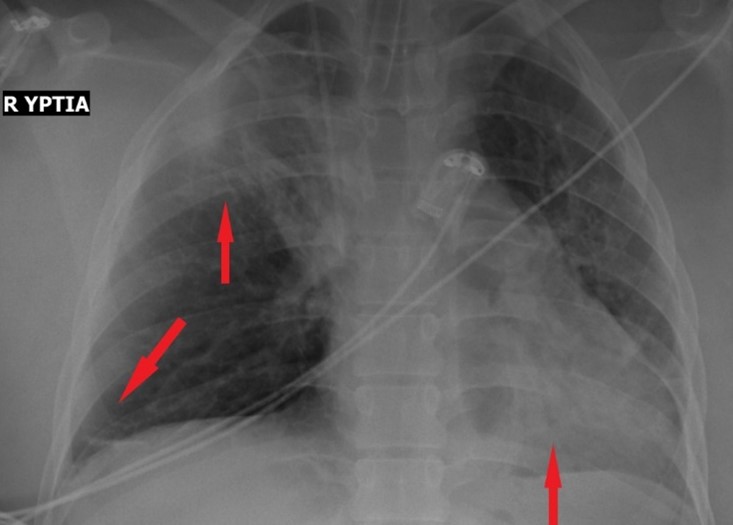
Figure 1: X-Ray of chest with atelectasis of upper right lobe and both of lower lobes.
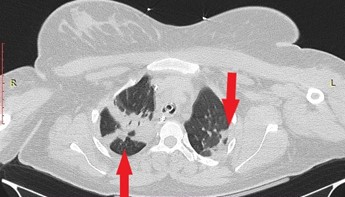
Figure 2: Computed Tomography (CT) of chest in axial plane with intravenous contrast. Atelectasis of both lower lobes.
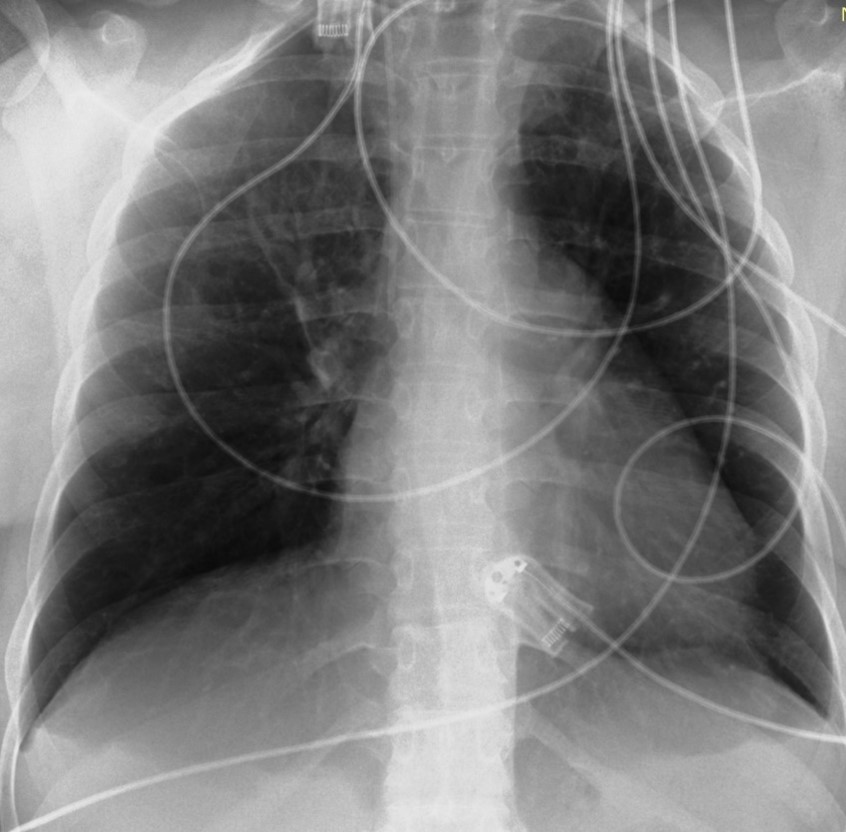
Figure 3: X-Ray of chest on the day of discharge from ICU (Intensive Care Unit). Normal.
Discussion
The management of severe preeclampsia constitutes to delivery of the fetus and removal of the placenta. Then, a close monitoring protocol is followed for the first 24 hours as it is the most critical period for the appearance of eclampsia. To prevent this risk at peripartum period and for 24 hours after delivery, the administration of an intravenous magnesium sulfate regimen is recommended [15]. Our patient was led into labor immediately after the appearance of signs of severe preeclampsia while she was also given intravenous magnesium sulfate to prevent eclamptic seizures. The intraoperative onset of respiratory failure was promptly managed but remained a differential diagnostic problem due to the acute onset and rapid establishment without signs of improvement.
Acute respiratory failure is a rare complication during pregnancy and the postpartum period affecting 1 in 500 pregnancies [16]. The onset of respiratory failure prepartum or postpartum could be attributed either to pre-existing respiratory problems or to severe pregnancy complications [13]. Our patient developed respiratory failure being intubated during the caesarean section. From her individual medical history there were no risk factors for burdening the respiratory system, for example asthma or pneumonitis. The intubation was done smoothly without any difficulties. The severe bronchospasm after intubation was transient, however, the patient developed respiratory failure with high oxygen requirements as evidenced by the first arterial blood gas test (PO2 106 with FiO2 100% when in a standard operating room FiO2 is about 45%) and lactic acidosis. Clinical and imaging examination ruled out major complications of brain, heart and lungs with the only finding of lung atelectasis.
In the search of the literature, several cases of respiratory failure in pregnancy or in the postpartum period were found, but in all cases, there was either an underlying disease or serious complications of preeclampsia such as pulmonary edema, perinatal cardiomyopathy etc. appeared and were diagnosed [17]. In the case of our patient, no complications were found and her medical history was free. Based on the pathophysiology of preeclampsia the patient's respiratory failure could be explained by the presence of airway edema, tissue hypoxia, increased endothelial permeability with the appearance of generalized peripheral edema and severe lactic acidosis as a result of tissue hypoxia that worsened her burdened respiratory profile.
Conclusion
Preeclampsia as a multifactorial and multiorgan disease has been described since the time of Hippocrates [18] and to this day is one of the main causes of maternal mortality and mortality even in the developed world. Despite all preventive measures, close maternal and fetal monitoring, and application of medication protocols, delivery remains the only treatment for preeclampsia. The challenge for obstetricians is to make the decision to terminate the pregnancy weighing the benefits to both the health of the mother and the newborn. Predicting complications in the mother is impossible most of the time as the disease has an unpredictable progress and outcome in previously hemodynamically and respiratoryly stable patients. Understanding the pathophysiology of the disease leads to prompt recognition of its complications, rapid differential diagnosis and management once the cause of the disease, the incompetent placenta, has been removed.
Funding: There was no Funding
Ethical Statements: All authors declare that they have no conflict of interest
Author’s Contributions: Maria Ntioudi participated in conceptualization, investigation, methodology, writing and original drafting the manuscript. Sophia Gelatou, Viki Karypidou and Dimitra Alatzidou were responsible for investigation, methodology and reviewing the paper. Thomas Karagkiouzis participated in supervision, reviewing and validating the final form.
References
- Hypertension G. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol, 2019; 133(1): e1–25.
- Steegers EAP, Von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet, 2010; 376(9741): 631–644.
- Leone M, Einav S. Severe preeclampsia: what’s new in intensive care? Intensive Care Med, 2015; 41(7): 1343–1346.
- Knight M, Bunch K, Tuffnell D, Patel R, Shakespeare J, Kotnis R, et al. Saving lives, improving mother’s care report. Midwifery, 2015; 31(2): 262–263.
- Homer CSE, Brown MA, Mangos G, Davis GK. Non-proteinuric pre-eclampsia: A novel risk indicator in women with gestational hypertension. J Hypertens, 2008; 26(2): 295–302.
- Steven A. Friedman, Robert N. Taylor JMR. Pathophysiology of preeclamsia. Clin Perinatol, 1991; 18(4): 661-682.
- Benedetti TJ, Kates R, Williams V. Hemodynamic observations in severe preeclampsia complicated by pulmonary edema. Am J Obstet Gynecol, 1985; 152(3): 330–334. http://dx.doi.org/10.1016/S0002-9378(85)80222-2
- Brichant J, Brichant G, Dewandre P, Foidart J. ´ e Manifestations he Circulatory and respiratory problems in preeclampsia. Ann Fr Anesth Reanim, 2010; 29(4): e91–95. http://dx.doi.org/10.1016/j.annfar.2010.02.023
- Peguero A, Alonso R, Paola S, Rojas-suarez J. Association of plasma lactate concentration at admission of severe preeclampsia to maternal complications. Pregnancy Hypertens. 2019; 17(October 2018): 89–93. https://doi.org/10.1016/j.preghy.2019.05.003
- Neligan PJ, Laffey JG. Clinical review: Special populations - critical illness and pregnancy. Crit Care, 2011; 15(4): 1–10.
- Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): Much ado about nothing? Am J Obstet Gynecol, 1990; 162(2): 311–316. http://dx.doi.org/10.1016/0002-9378(90)90376-I
- Pollock W, Rose L, Dennis CL. Pregnant and postpartum admissions to the intensive care unit: A systematic review. Intensive Care Med, 2010; 36(9): 1465–1474.
- Lapinsky SE. Acute respiratory failure in pregnancy. Obstet Med, 2015; 8(3): 126–132.
- Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynecol Obstet, 2019; 145(S1): 1–33.
- Lam M, Dierking E. Intensive Care Unit issues in eclampsia and HELLP syndrome. Int J Crit Illn Inj Sci, 2017; 7(3): 136–141.
- Lapinsky SE. Management of Acute Respiratory Failure in Pregnancy. Vol. 38, Seminars in Respiratory and Critical Care Medicine, 2017.
- Randhawa JS, Ashraf H, Colombo JP, Kudla P. Postpartum Respiratory Distress Due to Hypertension-Related Pulmonary Edema. Cureus, 2021; 13(9): 10–14.
- Bell MJ. A historical overview of preeclampsia-eclampsia. JOGNN - J Obstet Gynecol Neonatal Nurs, 2010; 39(5): 510–518.

