Esketamine in the Treatment of Depression with Co-Morbid Anorexia Nervosa
Rebecca Perrain, Lila Mekaoui and Philip GORWOOD*
CMME, Hôpital Sainte-Anne, GHU Paris Psychiatrie et Neuroscience, France
Received Date: 11/01/2024; Published Date: 23/05/2024
*Corresponding author: Philip Gorwood, MD, PhD, Department of Neurology, CMME, Hôpital Sainte-Anne, GHU Paris, 1 rue Cabanis, 75013 Paris, France
Introduction
Over the last thirty years, an excess of major depressive episodes (MDE) has been observed in patients with Anorexia Nervosa (AN). The lifetime prevalence of MDD in restrictive AN has been estimated to be between 9.5% and 64.7%, and in some studies as high as 75% in some studies [1,2]. There is evidence of a significant association between the severity of eating disorder symptoms and the importance of depressive symptoms [3], with the co-occurrence of MDD and AN identified as an additional risk factor for suicide attempts [4]. As a recent meta-analysis found no efficacy of antidepressant for depressive disorders in patients with anorexia nervosa [5], it is crucial to test for other approaches. In this regard, the efficacy of ketamine and its enantiomers in the treatment of depression associated with anorexia nervosa is of potential interest, but remains unknown. In this context, we describe a case of successful adjunctive esketamine treatment in a patient with AN associated with MDE.
Case Report
An 18-year-old female patient presented with a depressed mood and suicidal ideation with concurrent anorexia nervosa. She was diagnosed with Anorexia Nervosa (AN) according to DSM-5 during a face-to-face clinical assessment in a specialized centre. AN started in 2015 with a 5 months full time hospitalisation and naso-gastric tube feeding in 2019. Lifetime minimal weight was 37.5kg (BMI = 12.5) and lifetime maximal weight was 52kg (BMI = 17.6). Secondary amenorrhea started when the patient was xx old.
She was referred to an emergency department with a depressed mood and severe suicidal risk (suicidal attempt by defenestration). She was first prescribed 30mg mianserine during 3 weeks in March 2019, without clinical response. Olanzapine 5mg was prescribed from July to December 2019, based on previous studies highlighting weight and obsessive thoughts improvement in patients with anorexia nervosa treated with olanzapine [6-8].
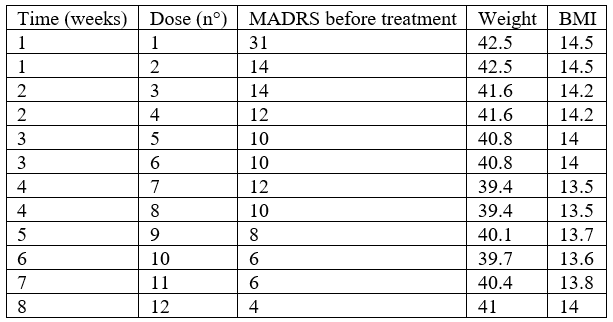
She had a second hospitalisation in our department xx months later, required by depressive symptoms. Psychomotor retardation, hypomimia, sadness, anhedonia, aboulia, and suicidal thoughts were still observed. There was no sleep disturbance nor psychotic feature. Twenty milligrams of paroxetine were therefore administered. No clinical response was observed, and paroxetine was cross-tapered with 30 mg of mirtazapine once daily over the course of 10 days and with adjunctive intranasal esketamine 56mg administered twice weekly during 4 weeks. Clinical response was observed since the first administration. Suicidal ideation disappeared (Table 1). Remission was reached at the beginning of the fifth week. Six months after the initiation of the treatment, the patient was still euthymic with the continuation of mirtazapine.
Table 1: MADRS, weight and BMI throughout treatment.
Discussion
Depression and anorexia nervosa are often co-morbid. According to a recent meta-analysis [5], there is no evidence for the effectiveness of antidepressants among patients with AN in terms of depressive symptoms regardless of the type of prescribed treatment (tricyclics, Selective Serotonin Reuptake Inhibitors, other antidepressants as monoamine oxydase inhibitors, venlafaxine). This meta-analysis does not mention ketamine nor esketamine because of the rarity of cases. The authors conclude that these psychotropic drugs should not be prescribed in AN.
An explanation for this problematic antidepressants’ ineffectiveness could be linked to malnutrition [9]. Several authors hypothesised that undernutrition and its consequences lead to depression and to treatment-resistance issues.
Abnormalities in central nervous system monoamine metabolisms were highlighted [10]. Some hypotheses are related to the lack of dietary tryptophan an essential amino acid, which is the precursor of serotonin (5-HT). Some studies indeed found a reduced level of 5-HT metabolite in the cerebrospinal fluid of animals and healthy humans under a restricted diet [9,10]. A few years later, in a simultaneous exploration of depression, nutritional status and the peripheral metabolism of tryptophan and serotonin, before and after re-feeding and weight gain, some authors gave further evidence that tryptophan availability could restore serotonin neurotransmission and lead to depression improvement after re-feeding [11]. Impaired serotonin pathway could also be triggered by vitamins dietary deficiency such as B6 or B12 vitamins, both being involved in 5-HT synthesis [9].
Another possible explanation is the lack of zinc, a mineral obtained from dietary sources (especially red meat, poultry, fish, and dairy products). Zinc is an allosteric modulator that physiologically blocks post-synaptic glutamatergic NMDA receptor functioning [9] and zinc levels are known to be higher in limbic structures as in the amygdala, hippocampus and across the cortex [12]. Low zinc levels are associated with treatment resistance due to malnutrition in AN [8] and were also linked to the risk of major depression [12,13,14]. At a cellular level, the lack of zinc leads to modified glutamatergic neurotransmission via increased NMDA receptor activity in several ways, including (1) decreased stimulation of GABA interneurons -which inhibits glutamate release from presynaptic neuron – leading to increased glutamate level in the synaptic cleft; (2) decreased inhibition of NMDAr, leading to increased levels of Calcium into post-synaptic neuron; and (3) decreased inhibition of metabotropic glutamate receptor, leading to an increased release of Calcium. Those effects, all together, result in an upregulation of NMDAr, that leads to an increased Calcium influx into the postsynaptic neurons, which can cause excitotoxicity, and therefore impair synaptogenesis and synaptic plasticity [13]. Zinc supplementation alone did not lead to AN and/or MDD remission, but adjunctive supplementation could be a helpful therapeutic strategy for MDD [5,12].
By inhibiting NMDA receptor, ketamine and esketamine could lead to “normal” state and avoid excitotoxicity. Ketamine and its enantiomer S-ketamine are NMDAr antagonists and have better biodisponibility when administered intravenously (100% biodisponibility) or by nasal spray (45% biodisponibility) compare to sublingual (30%) or oral (20%) [15]. Ketamine is now an acknowledged treatment for unipolar and bipolar depression and esketamine recently obtained FDA approval for treatment-resistant unipolar depression [15].
Its rapid antidepressant action contrasts with usual antidepressant treatments. Moreover, its specific action on suicidal ideation makes ketamine a particularly useful treatment to prevent suicidal behaviours in patients with anorexia nervosa who have one of the highest suicidal risk of death (Chesney et al., 2014).
A review assessed potential mechanisms of this rapid pharmacological action: AMPAr activation, NMDAr inhibition-mediated mechanisms, inhibition of NMDARs expressed on GABAergic interneurons (disinhibition hypothesis), inhibition of spontaneous NMDARmediated transmission, direct inhibition of extra-synaptic NMDAR [16]. Moreover, ketamine administration induces a rapid increase in total BDNF protein levels, within 30 minutes of administration. Thanks to its direct glutamatergic effect, ketamine might be efficient independently of nutritional state. Interestingly, there is convergent evidence that patients with anorexia nervosa have an excess of the Met-allele, the low activity BDNF genetic variant (Ribasés et al., 2005 ; Gratacòs et al., 2007; Mercader et al., 2007 ; Ceccarini et al., 2019), and BDNF serum concentrations are significantly higher in recovered AN participants compared to acute AN patients (Zwipp et al., 2014). Furthermore, intravenous or intranasal galenic allow better compliance avoiding lack of efficacy due to purgative behaviours.
Conclusion
Esketamine could be an interesting antidepressant treatment for patients suffering for AN with malnutrition, due to (1) the poor efficacy of conventional antidepressants in this population (), (2) the role of BDNF in both the pathophysiology of anorexia nervosa and mechanism of action of ketamine derived molecules, (3) the high risk of suicide in this population, and (4) the intranasal galenic which bypass the problem of purgative behaviours frequently observed in patients with anorexia nervosa. Controlled trials are therefore highly needed to compare esketamine or ketamine versus SSRI to treat depression in AN.
Conflict of interests: Philip Gorwood received during the last 5 years fees for presentations at congresses or participation in scientific boards from Alcediag-Alcen, Angelini, GSK, Janssen, Lundbeck, Otsuka, SAGE and Servier.
References
- Godart NT, Perdereau F, Rein Z, Berthoz S, Wallier J, Jeammet P, et al. Comorbidity studies of eating disorders and mood disorders. Critical review of the J Affect Disord, 2007; 97: 37–49. https://doi.org/10.1016/j.jad.2006.06.023
- Woodside BD, Staab R. Management of Psychiatric Comorbidity in Anorexia Nervosa and Bulimia Nervosa. CNS Drugs, 2006; 20: 655–663. https://doi.org/10.2165/00023210-200620080-00004
- Herpertz-Dahlmann BM, Remschmidt H. Depression in anorexia nervosa at follow- Int J Eat Disord, 1993; 14: 163–169. https://doi.org/10.1002/1098- 108x(199309)14:2<163::aid-eat2260140206>3.0.co;2-y
- Foulon C, Guelfi JD, Kipman A, Adès J, Romo L, Houdeyer K, et al. Switching to the bingeing/purging subtype of anorexia nervosa is frequently associated with suicidal attempts. European Psychiatry, 2007; 22: 513–519. https://doi.org/10.1016/j.eurpsy.2007.03.004
- Blanchet C, Guillaume S, Bat-Pitault F, Carles M-E, Clarke J, Dodin V, et al. Medication in AN: A Multidisciplinary Overview of Meta-Analyses and Systematic J Clin Med, 2019; 8. https://doi.org/10.3390/jcm8020278
- Bissada H, Tasca GA, Barber AM, Bradwejn J. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: a randomized, double-blind, placebo-controlled trial. The American Journal of Psychiatry, 2008; 165: 1281–1288. https://doi.org/10.1176/appi.ajp.2008.07121900
- Jensen VS, Mejlhede Anorexia nervosa: treatment with olanzapine. The British Journal of Psychiatry: The Journal of Mental Science, 2000; 177: 87. https://doi.org/10.1192/bjp.177.1.87
- La Via MC, Gray N, Kaye WH. Case reports of olanzapine treatment of anorexia The International Journal of Eating Disorders, 2000; 27: 363–366. https://doi.org/10.1002/(sici)1098-108x(200004)27:3<363::aid-eat16>3.0.co;2-5
- Barbarich NC, McConaha CW, Halmi KA, Gendall K, Sunday SR, Gaskill J, et al. Use of nutritional supplements to increase the efficacy of fluoxetine in the treatment of anorexia Int J Eat Disord, 2004; 35: 10–15. https://doi.org/10.1002/eat.10235
- Kaye WH, Ebert MH, Raleigh M, Lake R. Abnormalities in CNS monoamine metabolism in anorexia nervosa. Arch. Gen. Psychiatry, 1984; 41: 350–355. https://doi.org/10.1001/archpsyc.1984.01790150040007
- Gauthier C, Hassler C, Mattar L, Launay J-M, Callebert J, Steiger H, et al. Symptoms of depression and anxiety in anorexia nervosa: Links with plasma tryptophan and serotonin Psychoneuroendocrinology, 2014; 39: 170–178. https://doi.org/10.1016/j.psyneuen.2013.09.009
- Petrilli MA, Kranz TM, Kleinhaus K, Joe P, Getz M, Johnson P, et al. The Emerging Role for Zinc in Depression and Psychosis. Front. Pharmacol, 2017; https://doi.org/10.3389/fphar.2017.00414
- Hermens DF, Simcock G, Dutton M, Bouças AP, Can AT, Lilley C, et al. Anorexia nervosa, zinc deficiency and the glutamate system: The ketamine Prog. Neuropsychopharmacol. Biol. Psychiatry, 2020; 101: 109921. https://doi.org/10.1016/j.pnpbp.2020.109921
- Szewczyk B, Kubera M, Nowak G. The role of zinc in neurodegenerative inflammatory pathways in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, The Neuro-inflammatory and Neuroprogressive Pathways in Depression, 2011; 35: 693–701. https://doi.org/10.1016/j.pnpbp.2010.02.010
- Matveychuk D, Thomas RK, Swainson J, Khullar A, MacKay M-A, Baker GB, et al. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther Adv Psychopharmacol, 2020; 10: https://doi.org/10.1177/2045125320916657
- Zanos P, Gould TD. Mechanisms of Ketamine Action as an Antidepressant. Mol Psychiatry, 2018; 23: 801–811. https://doi.org/10.1038/mp.2017.255

