Heterogeneous Tumor Response to PD-1 Plus TKI Strategy with Rare Skin Metastases
Zhiyuan Zhang1,2,3,4, Xian Wu1,2,3,4, Yongsheng Yang5, Heng Wang2,6, Yang Wang2,6, Zhen Zhang1,2,3,4, Lijun Shen1,2,3,4,* and Fan Xia1,2,3,4,*
1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, China
2Department of Oncology, Shanghai Medical College, Fudan University, China
3Shanghai Clinical Research Center for Radiation Oncology, China
4Shanghai Key Laboratory of Radiation Oncology, China;
5Department of Dermatology, Affiliated Huashan Hospital, Fudan University Shanghai, China
6Nursing Department, Fudan University Shanghai Cancer Center, China
Received Date: 03/01/2024; Published Date: 22/05/2024
*Corresponding author:
1. Prof. Fan Xia, Department of Radiation Oncology, Fudan University Shanghai, Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University; Shanghai Clinical Research Center for Radiation Oncology; Shanghai Key Laboratory of Radiation Oncology, Shanghai 200032, China
2. Prof. Lijun Shen, Department of Radiation Oncology, Fudan University Shanghai, Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University; Shanghai Clinical Research Center for Radiation Oncology; Shanghai Key Laboratory of Radiation Oncology, Shanghai 200032, China
Zhiyuan Zhang and Xian Wu contributed equally to this work
Abstract
Metastatic colon and rectal cancer present significant hurdles in the cancer treatment. The combination of PD-1 inhibitors and TKIs holds immense potential for MSS tumors. However, the varying treatment responses and adverse effects associated with this approach necessitate further elucidation. We present a case of metastatic rectal cancer where the patient exhibited diverse responses to PD-1 plus TKI therapy. Contrasting outcomes were observed across different organs, including the occurrence of rare cutaneous metastasis. The inconsistent manifestations on the skin add complexity to comprehending medication side effects. Our findings highlight the necessity of differential diagnosis and vigilance regarding rare metastasis when considering new treatment strategies. Variations in immune microenvironments might contribute to the disparities in treatment responses. Additional research is crucial to optimizing outcomes in immunotherapy-based approaches.
Keywords: Case report; Colon and rectal cancer; Metastasis; Skin metastases; Programmed death-1 antibody; Tyrosine kinase inhibitors; Tumor heterogeneity
Abbreviations: CRC - Colon and Rectal Cancer; PD-1 - Programmed Death-1; MSI-H - Microsatellite Instability High; MSS - Microsatellite Instability Stable; TKI - Tyrosine Kinase Inhibitor; VEGFR - Vascular Endothelial Growth Factor Receptor; MSS - Microsatellite Instability-Stable; CRC - Colon-Rectal Cancer; AE - Adverse Effect; OS - Overall Survival; HFSR - Hand-Foot Skin Reaction; RCCEP - Reactive Cutaneous Capillary Endothelial Proliferation
Introduction
Immunotherapy has revolutionized cancer treatment by offering new possibilities for patients. While the success of Programmed Death-1 (PD-1) antibodies in Microsatellite Instability-High (MSI-H) Colon and Rectal Cancer (CRC) is evident, their effectiveness in Microsatellite Instability-Stable (MSS) patients remains challenging. To explore better treatment options, combining PD-1 antibodies with Tyrosine Kinase Inhibitors (TKIs) has shown promise in MSS tumors.
However, inconsistent treatment outcomes among metastatic sites can complicate clinical decision-making, reflecting the tumor's heterogeneity. The Regonivo study demonstrated higher response rates in lung metastases compared to liver metastases, highlighting the complexities in evaluating treatment effects and selecting interventions [1].
In summary, the response patterns and adverse effects of combined PD-1 antibodies and TKIs need further investigation. Understanding the heterogeneous nature of metastases and accumulating case reports are crucial for optimizing treatment outcomes and managing rare metastatic sites, including cutaneous metastases.
Case Presentation
In this case, we present the details of a 53-year-old male patient diagnosed with multi-metastasis rectal cancer. Following previous rounds of chemotherapy, the patient underwent laprascopic anterior resection and received five cycles of XELOX chemotherapy(Oxaliplatin and Capecitabine) after being diagnosed with rectal cancer on March 15, 2018. Pathological assessment revealed an uncertain T stage and positive lymph node involvement in all three nodes. Next-generation sequencing indicated a wild-type NRAS and MSS status. Unfortunately, in May 2019, metastasis was detected in the lymph node located at the groin, followed by a recurrence in the pelvis in May 2020. In September 2020, the patient experienced edema in the bilateral lower limbs and developed a hard nodule in the right groin. Further evaluation in February 2021 confirmed bone metastasis. Throughout this period, the patient received various lines of chemotherapy and targeted therapy, yet tumor progression persisted. The treatment regimens included oxaliplatin plus raltitrexed for one cycle, cis-platinum plus raltitrexed for four cycles, irinotecan for one cycle, irinotecan plus raltitrexed for three cycles, and docetaxel plus bevacizumab for one cycle. A timeline depiction of the case is provided in Figure 1.
Upon discovering edema in the patient's legs and metastasis in the right groin, a PET-CT scan revealed metastasis in both groins and multiple metastases in the bones (Figure 2 A-B). Subsequently, on November 16, 2020, the patient underwent three cycles of fruquintinib plus camrelizumab treatment. On January 13, 2021, a CT examination was conducted (Figure 2 C-D). A comparison with the previous assessment in May 2020 showed that the nodule in the right groin had grown to 3.5 cm*3.5 cm and had developed a fistula on the skin. However, there was partial alleviation of the edema and pain observed in the lower limbs. Given the increased size of the metastasis in the groin, the patient opted to undergo radiation therapy.
Due to the COVID-19 pandemic in China, the patient's access to radiotherapy was hindered. In the meanwhile, he continued with the original treatment strategy of fruquintinib plus camrelizumab until February 10, 2021. As the treatment progressed, a radiological image on February 24, 2021, revealed a significant reduction in the nodule in the right groin (Figure 2 E-F). The alleviation of the nodule obstruction resulted in partial relief of symptoms such as edema and pain after immunotherapy. However, during this period, the range of erythema on the skin gradually expanded. Based on the positive response to the fruquintinib plus camrelizumab treatment strategy, it was believed that the erythema appearing on the skin was closely associated with the adverse effects of PD-1 agents and TKI agents.
To mitigate the adverse effects of PD-1 and TKI drugs, the patient discontinued camrelizumab on February 10, 2021, and began the latest fruquintinib on January 25, 2021. Glucocorticoids were administered to manage the adverse events associated with immunotherapy. After discontinuing immunotherapy, the nodule in the groin showed sustained improvement, as depicted in the image on February 24, 2021 (Figure 2 E-F). However, the number and extent of erythema on the skin continued to worsen.
In order to achieve better tumor regression, the patient decided to undergo palliative radiotherapy for groin tumor control from February 24, 2021, to March 12, 2021, receiving a total dose of 50 Gy delivered in 25 fractions. After two weeks of treatment, there was partial regression of the lymph node, but the skin nodules accompanied by edema worsened without relief from itching and pain. As a result, the radiotherapy was discontinued on March 12, 2021, and the focus shifted to the management of the skin disorders.
The radiological diagnosis indicated that the lymph node was reduced significantly; however, the symptoms of skin and edema were exacerbated. With the treatment of immunotherapy and management of AE effects, the lymph node at the groin exhibited opposite clinical manifestations as the nodules of the skin. In consideration of the side effects of fruquintinib, we stopped fruquintinib starting on March 10, 2021. We requested a dermatological consultation on March 18, 2021. Nodules were exhibited in the right leg and waist (Figure 3 A-D), and the skin biopsy confirmed the metastasis, with limited amount of lymph cells (Figure 3 E-F). The immunohistochemical for skin biopsy can be seen in supplementary material (Supplementary Figure 1). After one week of steroid treatment for skin nodules and other symptomatic management, including improvement of vascular osmotic pressure and reduction of water retention, the pain became severe, and skin nodules appeared in the twist and abdomen. However, the situation of the patient was aggravated, and the T-2 image from March 24, 2021, is shown in Figure 2 G-H. Because of the poor response to steroid treatment, we suspected the nature of these skin nodules. Then, the patient received a skin biopsy for pathological confirmation. The pathological diagnosis indicated cutaneous metastases from colon-rectal cancer. After decreased steroids and continuous supportive treatment, the patient cooperated with our treatment with sincereness and trust. Finally, he left for a local hospital and died on April 2, 2021.
Following radiological assessment, significant reduction was observed in the lymph node, yet the symptoms of skin and edema worsened. Despite immunotherapy and effective adverse event management, the lymph node in the groin displayed conflicting clinical manifestations compared to the skin nodules. Considering the potential side effects of fruquintinib, its administration was ceased on March 10, 2021. A dermatological consultation was sought on March 18, 2021, which revealed the presence of nodules on the right leg and waist (Figure 3 A-D). Subsequent skin biopsy confirmed metastasis with limited lymph cell infiltration (Figure 3 E-F). Supplementary material (Supplementary Figure 1) displayed immunohistochemical analysis of the skin biopsy. Symptomatic management, including vascular osmotic pressure improvement and water retention reduction, along with one week of steroid treatment, was initiated for the skin nodules. Unfortunately, this approach resulted in severe pain and the emergence of nodules in the twist and abdomen. Consequently, the patient's condition deteriorated, as evidenced by the T-2 image on March 24, 2021 (Figure 2 G-H). Due to the inadequate response to steroid treatment, further investigation of the nature of these skin nodules was warranted. A subsequent skin biopsy was performed, confirming cutaneous metastases originating from colon-rectal cancer. Adjustments to the steroid dosage were made, and continuous supportive care was provided, with the patient demonstrating sincere cooperation and trust. Regrettably, the patient's condition worsened, necessitating transfer to a local hospital, where they passed away on April 2, 2021.
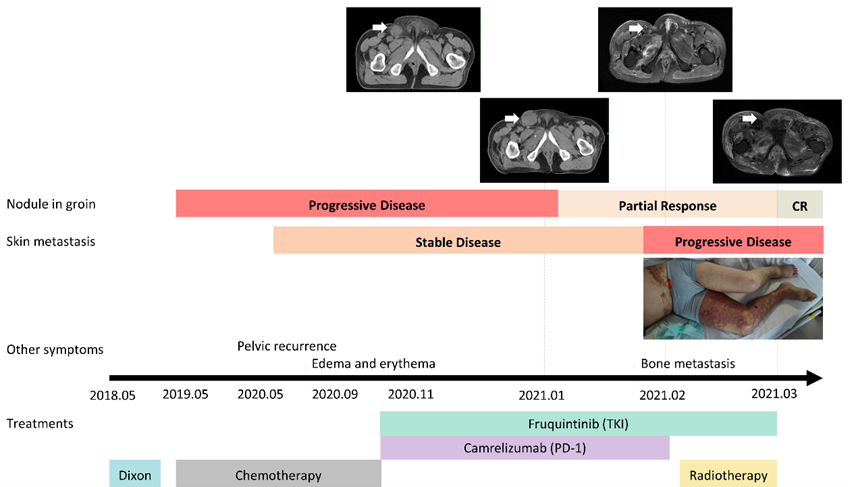
Figure 1: Representation of the process of tumor progression and treatments. The nodule in the groin and the skin metastasis exhibited the opposite clinical responses to the immune therapy.
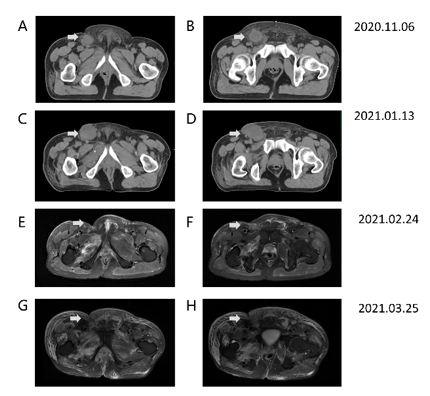
Figure 2: (A) and (B) show the CT for the localization of radiation therapy on 6th November 2020. The metastasis at the right groin can be seen. (C) and (D) represent the CT image of metastasis progression after PD-1 plus TKI for 3 cycles on 13th January 2021. A fistula from the right groin with a 3.5 cm*3.5 cm nodule could be palpated. However, the edema and pain were partly relieved. (E) and (F) show a T2-MRI image for the partial remission of metastasis from the right groin after PD-1 plus TKI on 24th February 2021, without radiotherapy. In addition, the patient received groin palliative radiotherapy as 50Gy/25Fx for tumor control. After 12 cycles of radiotherapy, he stopped radiation because of the aggressive pain and edema. (G) and (H) show the T2-MRI image of the situation on 24th March 2021, after the radiation. The metastasis of the groin showed nearly complete remission, but metastases in the skin and muscle exhibited opposite responses, resulting in persistent pain and edema.
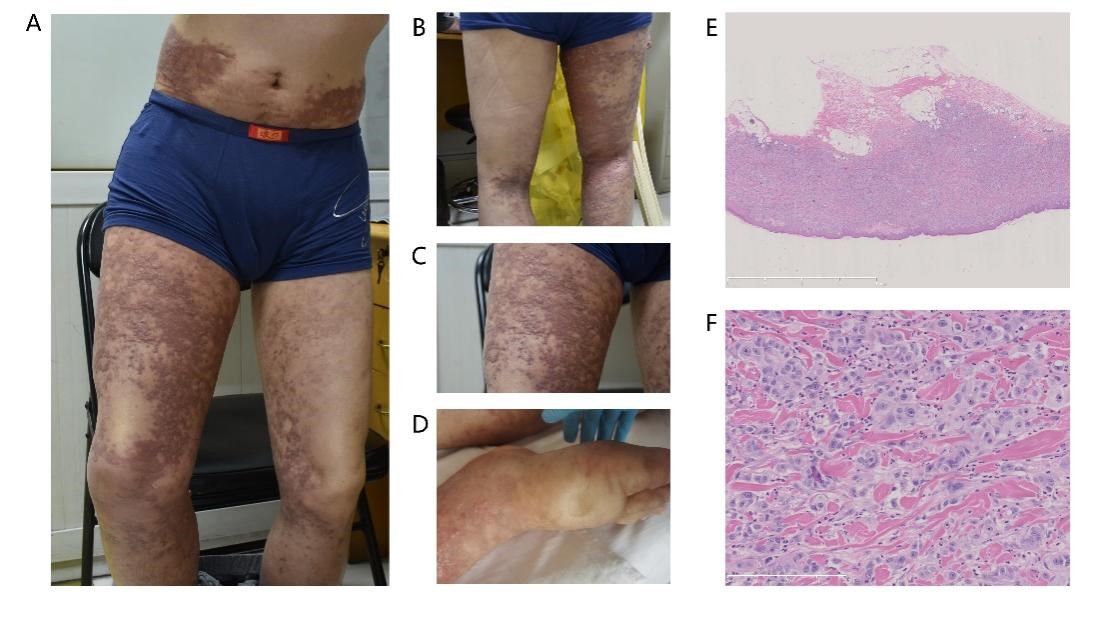
Figure 3: (A-C) shows the cutaneous metastases on the twist, which presented as nodules and pigmentation of the trunk. (D) shows the pitting edema of the right foot. (E-F) exhibits the pathological biopsy with 2x and 20x magnification.
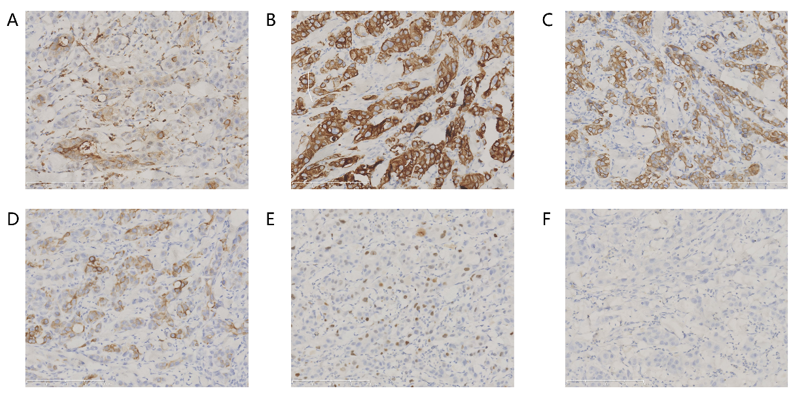
Supplementary Figure S1: (A) represented the immunohistochemistry (IHC) for CEA. (B) represented the IHC for CK7. (C) represented the IHC for CK8. (D) represented the IHC for CK20. (E) represented the IHC for Ki67. (F) represented the IHC for P63.
Discussion
Heterogeneity in cancer progression and treatment is complex, involving diverse intratumor cells and microenvironments that shape therapy responses. In one case study, melanoma metastases from different sites exhibited distinct genomic alterations and immune microenvironment characteristics, suggesting individual variations and immune responses in each metastatic lesion [2]. The organ-specific environment also plays a crucial role in shaping the tumor microenvironment, influencing immunotherapy outcomes. For example, organ-specific agents can stimulate distinct functional statuses in regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), thereby influencing their immunosuppressive activities [3,4].
Clinical experiences demonstrate differences in immune environments among organs and varying effects of immunotherapy on different organs. Response rates to immunotherapy have been observed to differ between metastases in the liver and lung, highlighting organ-specific variations[1]. Clinical trials investigating immunotherapy have also shown varying effects on different organs [5,6]. Therefore, it is necessary to explore suitable local and systemic treatment approaches based on the immune characteristics of different organs.
In our case, differential responses to immunotherapy were observed among metastatic tumors, indicating diverse immune environments within these lesions. Understanding these complexities is crucial as immunotherapy with resistance and tolerance to immunotherapy are important considerations in clinical practice. Sharing this case report aims to contribute to improving insights into this transformative treatment approach among researchers and physicians.
Declaration of conflicting interests: We have no conflicts of interest to disclose.
Acknowledgments: We thank all the subjects who volunteered to participate in this study and the patient fighting cancer.
Authors’ Contributions: Treatment decision: Zhen Zhang and Fan Xia. Acquisition, analysis, and interpretation of data: All authors. Drafting of the manuscript: Zhiyuan Zhang and Fan Xia. Critical comment on the manuscript: Zhiyuan Zhang, Fan Xia and Lijun Shen.
Funding:
Beijing Xisike Clinical Oncology Research Foundation (Y-Young2022--0278)
Shanghai Municipal Health commission (20214Y0146)
National Natural Science Foundation of China (82003229)
Shanghai Anticancer Association (HYXH2021096)
Data availability statement: The data underlying this article may be shared upon reasonable request to the corresponding author, following approval from the involved Research Institutions.
References
- Fukuoka S, Hara H, Takahashi N, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603) J. Clin. Oncol, 2020; 38: 2053-2061.
- Liu D, Lin J-R, Robitschek EJ, et al. Evolution of delayed resistance to immunotherapy in a melanoma responder Nat. Med, 2021; 27: 985-992.
- Huppert LA, Green MD, Kim L, et al. Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy Cell. Mol. Immunol, 2021; DOI: 10.1038/s41423-021-00742-4:10.1038/s41423-021-00742-4
- Guha P, Gardell J, Rabinowitz B, et al. Monocytic and granulocytic myeloid-derived suppressor cell plasticity and differentiation are organ-specific Oncogene, 2021; 40: 693-704.
- Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination Nat. Med, 2021; 27: 152-164.
- Ho WJ, Erbe R, Danilova L, et al. Multi-omic profiling of lung and liver tumor microenvironments of metastatic pancreatic cancer reveals site-specific immune regulatory pathways Genome Biol, 2021; 22: 154.

