Acquired Immunodeficiency Syndrome Cholangiopathy in the Highly Active Antiretroviral Therapy Era
Rohit Nathani1,*, Bo Hyung Yoon2 and Ilan Weisberg2
1Department of Internal Medicine, Icahn School of Medicine at Mount Sinai, Mount Sinai Morningside-West Hospital, USA
2Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, Mount Sinai Beth Israel-Morningside-West Hospital, USA
Received Date: 29/12/2023; Published Date: 15/05/2024
*Corresponding author: Rohit Nathani, MD, Department of Internal Medicine, Icahn School of Medicine at Mount Sinai, Mount Sinai Morningside-West Hospital, New York, NY, USA
Abstract
Acquired Immunodeficiency Syndrome (AIDS) cholangiopathy is a clinical syndrome characterized by a cholestatic pattern of elevated liver chemistries, abdominal pain, and typical cholangiographic findings. Although quite common in the pre-Highly Active Antiretroviral Therapy (HAART) era, this condition is now rarely seen. It is important to be familiar with clinical presentation, etiology, diagnosis, and management of this condition as it is associated with a high mortality. We present a case of AIDS cholangiopathy in a young man who was non-adherent with HAART.
Introduction
First described in 1983 by Pitlik et al [1] and Guarda et al [2], Acquired Immunodeficiency Syndrome (AIDS) cholangiopathy (AC) is now a well-defined clinical entity characterized by right upper quadrant abdominal pain, biochemical abnormalities especially elevated Alkaline Phosphatase (ALP) and classic imaging findings. Although quite common in developing countries, the incidence of this disease has decreased in the West with the introduction of highly active antiretroviral therapy (HAART) [3]. It is important to identify AC due to its high mortality [4]. The following is a case of asymptomatic AC presenting with an elevated ALP, which was incidentally noted.
Case
A 37-year-old man with AIDS, who was not adherent with HAART, presented to the emergency department with burning upper back pain for 2 weeks. His vital signs were stable, and his physical examination was remarkable for a thin African American male in no acute distress with blistering and ulcerative skin lesions on the upper back. Abdominal examination revealed a soft, non-tender abdomen. The remainder of the physical examination was unremarkable. The lesions were thought to be zoster-related, and he was treated with valacyclovir and initiated on HAART and trimethoprim/sulfamethoxazole prophylaxis. His hospital course was complicated by sepsis secondary to soft tissue infection of the wound for which he underwent incision and drainage and was treated with antibiotics.
On initial presentation, he was incidentally noted to have an elevated ALP to 1739. He denied any abdominal pain, nausea, vomiting, diarrhea, constipation, jaundice, changes in the color of stools or urine, pruritus. He denied any alcohol use but did admit to using amphetamine, marijuana, and tobacco. He did not report any herbal supplement use or recent travel.
Other labs were remarkable for aspartate aminotransferase 232 IU/L, alanine aminotransferase 95 IU/L, total bilirubin 1.1 mg/dl, with direct of 0.2 mg/dl, gamma-glutamyl transferase (GGT) 873 IU/L, CD4 count of 4 and human immunodeficiency virus (HIV) viral load of 3,000,000. Ultrasound of the abdomen showed hepatomegaly with increased echogenicity of the hepatic echotexture. Magnetic resonance cholangiopancreatography (MRCP) revealed mild intra and extra hepatic biliary ductal dilatation and common bile duct measuring 1.2 cm, without evidence of gallstones or gallbladder wall thickening (Figure 1).
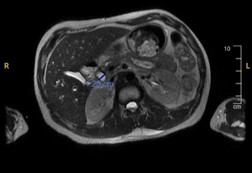
Figure 1
Infectious workup revealed immunity to hepatitis B and was negative for other hepatitis viruses. Epstein Barr Virus (EBV) and Cytomegalovirus (CMV) PCR were negative. QuantiFERON gold for Tuberculosis was negative. Autoimmune workup was negative for antinuclear antibodies and antimitochondrial antibodies; however, anti-smooth muscle antibodies were moderately elevated at 32. Given the differential of AC, a gastrointestinal stool pathogen panel was ordered and was positive for Cryptosporidium species and Enteropathogenic Escherichia coli.
A diagnosis of AC was made given the markedly elevated ALP and GGT with MRCP revealing intra and extra-hepatic biliary dilatation. Treatment with nitazoxanide for Cryptosporidium was initiated and he was offered a liver biopsy. The patient refused liver biopsy and was discharged with outpatient gastroenterology follow up.
Discussion
Opportunistic infections associated with immunosuppressed states have been implicated in the pathogenesis of AC. Cryptosporidium parvum was identified in our patient and is associated with 20-57% of AC cases [5]. It causes cholangiopathy via apoptosis of biliary epithelial cells through the Fas/FasL pathway, subsequently causing fibrosis which leads to biliary stricturing [6,7]. The second most implicated pathogen is CMV accounting for 10-20% of cases and is believed to cause vascular injury leading to ischemic cell death and fibrosis. Microsporidium, Mycobacterium Avium Complex, and other opportunistic infections have been inconsistently implicated [7].
The clinical presentation of AC can range from asymptomatic as was seen in our patient to severe right upper quadrant abdominal pain, diarrhea, fevers, jaundice. The degree of abdominal pain has been linked to the degree of papillary stenosis [8,9].
The classic laboratory abnormalities associated with AC are a markedly elevated GGT and ALP, as seen in our patient. Elevations in bilirubin and transaminases have also been reported inconsistently [9]. A study by Daly et al showed that ultrasound is 97% sensitive and 100% specific in diagnosing AC when compared to endoscopic retrograde cholangiopancreatography (ERCP) which is the gold standard [10]. Being cost effective, this is often the first imaging modality used [8,11]. MRCP is now preferred over ERCP if there is no indication of diagnostic or therapeutic procedures [12].
Narcotics such as opioids can provide symptomatic pain relief. The study by Cello et al showed that endoscopic sphincterotomy in AC with papillary stenosis provided sustained improvement in abdominal pain when followed up over a period of at least 9 months [8]. This is likely due to decompression of biliary ducts and bile drainage. The same study showed that sphincterotomy did not lead to a sustained decrease in ALP. For patients not responding to pain management with opioids and sphincterotomy, celiac plexus block has shown to have promising results [13].
A small study with four AC patients treated with ursodiol showed improvement in abdominal pain and a fall in ALP and GGT. However, no follow-up studies have been conducted [14].
Although other agents like paromomycin and azithromycin have been studied, nitazoxanide is the only drug approved by the United States Food and Drug Administration in the treatment of cryptosporidiosis. It is effective in reducing load of parasites in immunocompetent hosts; however, benefit in immunocompromised hosts is uncertain but often considered due to potentially serious complications [15].
Intravenous ganciclovir and foscarnet have not shown benefit in the treatment of CMV cholangitis. Similarly, treatments for other opportunistic pathogens have not shown consistent results [7]. Control of HIV with HAART has shown the most improvement in mortality in AC [4].
Although prior studies showed survival of 7-12 months, recent studies show increased survival to 34 months due to improved access and adherence to HAART [3]. The study by Ko et al found that the presence of opportunistic infections, especially cryptosporidium infection at the time of diagnosis and ALP >1000 was associated with poor outcomes [4]. CD4 count, the severity of cholangiopathy, and sphincterotomy for biliary decompression had no effect on mortality. Progression of sclerosis and cholangiocarcinoma are the complications that have been associated with AC and unfortunately progress despite treatment with HAART.
Our case highlights the important aspects in the etiology, diagnosis, management, and prognosis of AC – a condition associated with high morbidity and mortality. It also calls for more research in distinct aspects of this rarely seen diagnosis in the HAART era.
References
- Pitlik SD, Fainstein V, Garza D, Guarda L, Bolivar R, Rios A, et al. Human cryptosporidiosis: spectrum of disease. Report of six cases and review of the literature. Arch Intern Med, 1983; 143(12): 2269-2275.
- Guarda LA, Stein SA, Cleary KA, Ordonez NG. Human cryptosporidiosis in the acquired immune deficiency syndrome. Arch Pathol Lab Med, 1983; 107(11): 562-566.
- Devarbhavi H, Sebastian T, Seetharamu SM, Karanth D. HIV/AIDS cholangiopathy: clinical spectrum, cholangiographic features and outcome in 30 patients. J Gastroenterol Hepatol, 2010; 25(10): 1656-1660.
- Ko WF, Cello JP, Rogers SJ, Lecours A. Prognostic factors for the survival of patients with AIDS cholangiopathy. Am J Gastroenterol, 2003; 98(10): 2176-2181.
- Wilcox CM, Monkemuller KE. Hepatobiliary diseases in patients with AIDS: focus on AIDS cholangiopathy and gallbladder disease. Dig Dis, 1998; 16(4): 205-213.
- Chen XM, Gores GJ, Paya CV, LaRusso NF. Cryptosporidium parvum induces apoptosis in biliary epithelia by a Fas/Fas ligand-dependent mechanism. Am J Physiol, 1999; 277(3): G599-608.
- Naseer M, Dailey FE, Juboori AA, Samiullah S, Tahan V. Epidemiology, determinants, and management of AIDS cholangiopathy: A review. World J Gastroenterol, 2018; 24(7): 767-774.
- Cello JP. Acquired immunodeficiency syndrome cholangiopathy: spectrum of disease. Am J Med, 1989; 86(5): 539-546.
- Ducreux M, Buffet C, Lamy P, Beaugerie L, Fritsch J, Choury A, et al. Diagnosis and prognosis of AIDS-related cholangitis. AIDS, 1995; 9(8): 875-880.
- Daly CA, Padley SP. Sonographic prediction of a normal or abnormal ERCP in suspected AIDS related sclerosing cholangitis. Clin Radiol, 1996; 51(9): 618-621.
- Bilgin M, Balci NC, Erdogan A, Momtahen AJ, Alkaade S, Rau WS. Hepatobiliary and pancreatic MRI and MRCP findings in patients with HIV infection. AJR Am J Roentgenol, 2008; 191(1): 228-232.
- Tonolini M, Bianco R. HIV-related/AIDS cholangiopathy: pictorial review with emphasis on MRCP findings and differential diagnosis. Clin Imaging, 2013; 37(2): 219-226.
- Collazos J, Mayo J, Martinez E, Callejo A, Blanco I. Celiac plexus block as treatment for refractory pain related to sclerosing cholangitis in AIDS patients. J Clin Gastroenterol, 1996; 23(1): 47-49.
- Castiella A, Iribarren JA, Lopez P, Arrizabalaga J, Rodriguez F, von Wichmann MA, et al. Ursodeoxycholic acid in the treatment of AIDS-associated cholangiopathy. Am J Med, 1997; 103(2): 170-171.
- Abubakar I, Aliyu SH, Arumugam C, Hunter PR, Usman NK. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst Rev, 2007(1): CD004932.

