HbA1c of 51% in a 56-Year-Old Female with Axial Spondylarthritis
Christoph Winkler1,*, Raute Sunder3, Ines Peschel1, Andrea Griesmacher1 and Michael Schirmer2
1Department for Medical and Chemical Laboratory Diagnosis, Innsbruck University Hospital, Innsbruck, Austria
2Department of Internal Medicine, Clinic II, Innsbruck Medical University, Anichstraße 35, 6020 Innsbruck, Austria
3Department of Laboratory Medicine, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria
Received Date: 17/11/2023; Published Date: 23/04/2024
*Corresponding author: Christoph Winkler, Central Institute for Medical and Chemical Laboratory Diagnosis, Innsbruck University Hospital, Anichstraße 35, 6020, Innsbruck, Austria
Keywords: Hemoglobin variants; HbA1c; HPLC; DNA sequencing; Haemoglobin electrophoresis
Questions to Consider
- What are common known hemoglobin variants?
- Which laboratory methods can be used to separate the different hemoglobin fractions?
- Are there alternatives to Hba1c in long-term glucose monitoring?
Questions to Consider
- What are common known hemoglobin variants?
- Which laboratory methods can be used to separate the different hemoglobin fractions?
- Are there alternatives to Hba1c in long-term glucose monitoring?
Case Description
Hence, we present a falsely elevated HbA1c in a 56-year-old female patient. The patient presented to the rheumatology outpatient clinic because of a known axial spondylarthritis. When screening for comorbidities, routine diagnostics showed an implausibly elevated HbA1c of 51.1% whereas fasting blood glucose level and fructosamine were reported within the reference range. The remaining laboratory results are largely unremarkable.
Determination of HbA1c in EDTA blood was performed using high-performance liquid chromatography with a porous cation exchange column (Figure 1).
Diagnostics were extended by hemoglobin electrophoresis (HYDRASYS 2, Sebia, Lisses, France) and hemoglobin variant analysis by high pressure liquid chromatography (HPLC TOSOH G8).
Electrophoretic separation of hemoglobin is performed with the hemolysate of washed erythrocytes on agarose gels in alkaline (pH 8.5) and acidic (pH 6.0) environments. The hemoglobins are separated and stained with amido black staining solution. Based on the principle of high-performance liquid chromatography (HPLC), the analyzer uses a cation exchange column to separate the hemoglobin components by different ion interactions. The different hemoglobin components, including hemoglobin F and A2, are separated rapidly (6.0 min per sample). A combination of step gradient and linear gradient with three different salt concentrations (G8 β-Thalassemia Elution Buffer) is used for separation.
Analysis of hemoglobin variants by HPLC shows an abnormal band in the range between 1.51 min and 2.31min, but no evidence is found for the presence of any of the common hemoglobin variants (HbS, HbC, HbD, HbE). In Hb electrophoresis, in addition to HbA in the acidic environment, a distinct band was detected in the HbF region. In the alkaline milieu, no additional abnormal band was detected (Figure 2). However, HbF cannot explain the additional band due to a different running behaviour in the HPLC-based variant analysis.
After the patient gave informed consent for DNA analysis, sequencing and copy number analysis of the HBA1, HBA2 and HBB genes was performed and revealed a benign heterozygous variant (c.8A>T, p.His3Leu, Hb Graz) in the HBB gene. This very rare missense variant causes an amino acid exchange that does not affect the function of the beta globin chain, but leads to a deviating migration pattern in Hb electrophoresis and in the HPLC-based measurement of HbA1c.
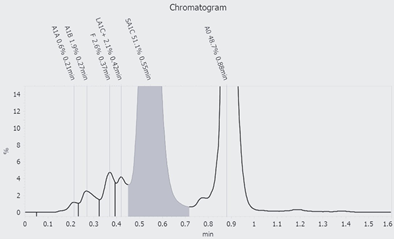
Figure 1: HbA1c analysis with HPLC: The chromatogram shows an abnormal hemoglobin of 51.1% which falls within the measurment range of HbA1c.
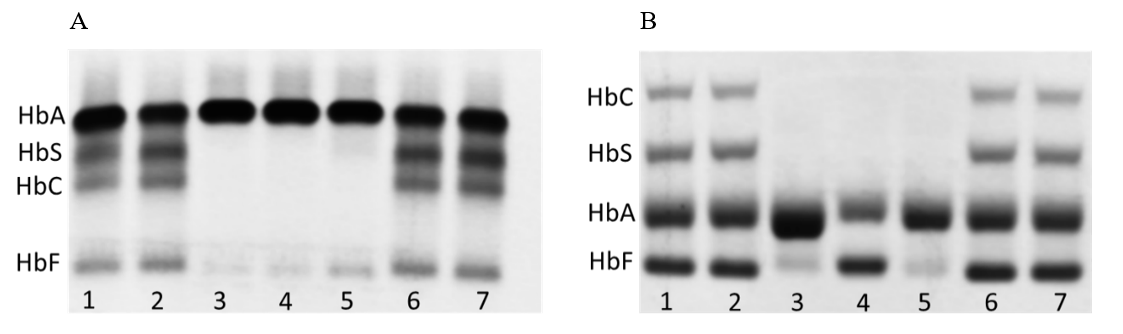
Figure 2: Hb Electrophoresis: the patient is found on lane 4, the controls on lane 1/2 and 6/7, and two normal patients on lane 3 and 5. (A) Hb Electrophoresis in alkaline milieu shows no additional bands.
(B) Hb Electrophoresis in acidic milieu shows an abnormal hemoglobin band in the region of HbF.
Discussion
HbA1c is a widely used diagnostic marker for the long-term assessment of glycemic control in patients with diabetes mellitus [1]. It reflects the average blood glucose levels over a period of approximately three months. HbA1c measurement is based on the glycation of the N-terminal valine residue in the β chain of hemoglobin [2]. Hemoglobin A1 is the primary hemoglobin component in human red blood cell hemolysate, accounting for 90% of the total protein. In addition, there are several minor hemoglobin variants (HbA2, HbF, HbA1a1, A1a2, A1b, and A1c) that can be determined by HPLC.
However, it is important to recognize that certain hemoglobin variants can interfere with the accurate measurement of HbA1c, leading to potentially incorrect results.
Hemoglobin variants are caused by genetic alterations that affect the structure and/or function of the hemoglobin molecule. Over 1,200 variants of hemoglobin have been identified, with the β-chain being involved in approximately 70% of cases [3]. While most variants are rare, some, such as HbS, HbC, HbD and HbE are relatively common in certain populations [4].
The presence of hemoglobin variants can be challenging for accurate quantification of HbA1c levels. These variants can affect the glycation process, alter the stability of HbA1c, or interfere with the methodologies used in its measurement. As a result, individuals with certain hemoglobin variants may exhibit falsely elevated or decreased HbA1c values, leading to potential misdiagnosis or inappropriate management of diabetes mellitus.
In this context, the determination of fructosamine in plasma may be an alternative to HbA1c measurement in patients with hemoglobinopathies.
In this case the identified heterozygous missense mutation in the HBB gene (c.8A>T, p.His3Leu) leads to the production of a variant beta globin chain called Hb Graz, which was initially discovered in 1993 in four related patients and their families in Austria [5]. Due to the scarcity of this hemoglobin variant, there are no data on the prevalence of Hb Graz. This benign Hb variant interferes with the HPLC based measurement of HbA1c and results in a falsely elevated HbA1c value.
To ensure accurate HbA1c measurement in the presence of hemoglobin variants, it is essential to identify and characterize these variants through specialized laboratory techniques. Hemoglobin electrophoresis and high-performance liquid chromatography (HPLC) are commonly employed methods to detect and differentiate various hemoglobin variants [6]. Additionally, genetic analysis of the individual hemoglobin genes can provide valuable insights into the specific hemoglobin gene mutations responsible for the observed interference.
In addition to the common variants HbS, C, E, and D, some rare hemoglobin variants are described in the literature that have a similar effect on the measurement of glycosylated HbA1c [7,8].
It is essential to recognize these hemoglobin variants in order to avoid a misdiagnosis and consequent damage to the patient. Laboratorians should be aware of their method's limitations and clinicians should question laboratory results that do not fit the clinical picture of the patient.
Acknowledgements: Thanks to Stadler Kristina, Huemer Theresia and Trojer Michaela for their support in the diagnostic work up of this patient.
Points to remember:
Over 1,200 hemoglobin variants exist, with some, like HbS, C, D, and E, being relatively common in certain populations.
Hemoglobin variants can interfere with the glycation process and measurement methodologies, potentially leading to falsely elevated or decreased HbA1c values.
Hemoglobin electrophoresis and HPLC are used to detect and differentiate hemoglobin variants, and genetic analysis can provide insights into specific mutations causing interference.
Some rare hemoglobin variants also affect HbA1c measurements, making it essential for healthcare professionals to be aware of these variants to avoid misdiagnosis.
Clinicians should question laboratory results that do not align with the patient's clinical condition, especially in cases involving known or suspected hemoglobin variants.
Conflict of interest: None declared.
Funding: None declared.
Patient consent: The patient has given written consent to the inclusion of material pertaining to herself; she acknowledged that she cannot be identified via the paper, and we have fully anonymised the case report.
Ethical approval: Not applicable.
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes care, 2021; 44: 15–33.
- Sacks DB, Bebu I, Lachin JM. Refining Measurement of Hemoglobin A1c. Clin Chem, 2017; 63: 1433-1435.
- Patrinos GP, Giardine B, Riemer C, Miller W, Chui DH, Anagnou NP, et al. Improvements in the HbVar database of human hemoglobin variants and thalassemia mutations for population and sequence variation studies. Nucleic Acids Res, 2004; 32: 537-541.
- Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World HealthOrgan, 2008; 86: 480–487.
- Liu JS, Molchanova TP, Gu LH, Wilson JB, Hopmeier P, Schnedl W, et al. Hb Graz or alpha 2 beta 2(2) (NA2) His-->Leu; a new beta chain variant observed in four families from southern Austria. Hemoglobin, 1992; 16: 493-501
- Old J, Harteveld CL, Traeger-Synodinos J, et al. Prevention of Thalassaemias and Other Haemoglobin Disorders: Volume 2: Laboratory Protocols. 2nd edition. Nicosia (Cyprus): Thalassaemia International Federation; 2012.
- Ao X, Ganta N, Choe S, Patel P, Turro J, Cheriyath P. Hemoglobin Wayne: A Rare Variant That Can Cause Falsely Elevated Hemoglobin A1c. Cureus, 2022; 14: 26559
- Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol, 2009; 3: 446-451.

