Complicated Atrioventricular Block Rheumatoid Arthritis Complete and Intracardiac Mass
Bouamoud Asmaa*, Bouazaze Marouane, Essanaa Badr, Alaoui Zahidi Hiba, Pr Amri Rachida and Pr Zarzur Jamila
Department of Medicine, University Mohammed V de Rabat, Morocco
Received Date: 12/10/2023; Published Date: 22/03/2024
*Corresponding author: Bouamoud Asmaa, Department of Medicine, University Mohammed V de Rabat, Morocco
Abstract
Rheumatoid Arthritis (RA) is a chronic autoimmune joint disease characterized by persistent systemic inflammation.
In rheumatoid arthritis, cardiac involvement is not uncommon and may involve all three tunics of the heart
We report an exceptional case of RA associated with complete Atrioventricular Block (AVB) and intracardiac mobile mass.
This is a patient with RA initially put on chloroquine, complicated by a complete BAV implanted with a double chamber pace, then put on corticosteroid therapy and methotrexate for four years. Faced with the worsening of his osteoporosis and the probable increase in the threshold of pacemaker stimulation, it was decided to replace methotrexate with leflunomide. As part of the patient's cardiac evaluation, an ETT was performed, inadvertently objectifying a mass in the right atrium attached to the atrial tube and the tricuspid valve without obstruction.
Full BAV is very rare. It can be seen especially in old polyarthritis. Several mechanisms can explain this, including rheumatoid nodules; Chloroquine poisoning; amyloid infiltration and rheumatoid vasculitis.
The association of RA and intracardiac mass is rare. By analyzing the few cases described in the literature, five main causes are summarized: rheumatoid nodules, cancers, granulomatosis of valves, intracavitary thrombus and infective endocarditis.
We discuss through our case, the epidemiological and etiological particularity of the association of RA with a major conductive disorder, as well as the difficulty of the etiological diagnosis of an intracardiac mass in the context of RA.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune joint disease characterized by persistent systemic inflammation. Patients present with joint pain and loss of physical ability; however, the prognosis depends mainly on cardiovascular events [1].
In rheumatoid arthritis, cardiac involvement is not exceptional and may involve all three tunics of the heart, particularly the pericardium, as shown by the first autopsy studies and confirmed by recent cardiographic echo studies [2]. Rheumatoid arthritis (RA) can cause valvular heart disease with some histological features similar to poststreptococcal rheumatic valve disease [3,4].
Case Report
This is a 42-year-old patient, carrier of RA since the age of 18 years initially put on chloroquine for 24 years, at the age of 38 she benefited from the implantation of a pacemaker in relation to a complete BAV, then put on corticosteroid therapy and methotrexate with discontinuation of chloroquine for 4 years. Faced with the worsening of his osteoporosis and the notion of episodes of lipothymia following methotrexate injections probably related to an increase in the threshold of pacemaker stimulation, it was decided to replace methotrexate with leleflunomide. As part of the patient's cardiac evaluation, echocardiography was performed 5 months after the start of leflunomide, incidentally objectifying a mass of 17x6 mm in the right atrium attached to the atrial tube and the tricuspid valve without hemodynamic obstruction. With a negative CRP and a normal white blood cell count and a series of blood cultures that came back negative. It was decided to put the patient on anticoagulant therapy in the face of the strong suspicion of a thrombus, however the mass did not change in size after two months of treatment. Faced with the onset of menometrorrhagia and the non-regression of the size of the mass, anticoagulation was stopped. After the medical-surgical staff, it was decided to perform a PET scan to rule out endocarditis and then propose surgery to excision the mass in view of the embolic risk, however the patient refused all therapeutic proposals.
Discussion
Rheumatoid arthritis is a predominantly synovial connective tissue disease, it is an inflammatory rheumatic disease causing joint deformities and destruction and a systemic disease, leading to extra-articular manifestations that can compromise the prognosis for life, such as heart damage [2].
Rheumatoid arthritis affects 1.5 million adults in the United States and 2 million in Europe [5]. Most cardiac involvement in RA remains asymptomatic and is described in 2% to 10% of patients[6].
Nodules are the only cardiac manifestation that is specific to RA, these nodules have the same histology as subcutaneous nodules [6].
Complete Atrioventricular Block (AVB) is, however, very rare. It can be observed especially in old, erosive and nodular polyarthritis. Ahern et al. [7] report an incidence of BAV in the range of 1 per 1000 patients followed for severe rheumatoid arthritis.
Complete atrioventricular block is a rare progressive event, Ahern et al. [7] reported 8 cases in 1983 and collected only 20 others in the literature. This disease occurs mainly in the case of advanced rheumatoid arthritis, several mechanisms can explain it, namely: rheumatoid nodules, chloroquine poisoning, amyloid infiltration and rheumatoid vasculitis [2].
Rheumatoid nodules are the most common cause and represent the most common extra-articular manifestation; they are part of the criteria of the American RheumatismAssociation. They are most often mobile or more rarely adherent, single, rounded or polylobed subcutaneous nodules 0.5 to 1 cm in diameter, mainly located on the posterior aspect of the forearm and elbow. Visceral localizations, particularly cardiac localizations, have been described. Rarely present at the beginning of the disease, they most often appear after several years of evolution. These rheumatoid nodules represent the only lesions specific to cardiac involvement in rheumatoid arthritis. The presence of these nodules in cardiac structures was first reported in 1944 by Baggenstoss and Rosenberg, these nodules were present in the aortic, mitral and pericardial valves. These nodules can affect all cardiac tissues with variable clinical expression depending mainly on their location, their severity and the associated non-specific inflammatory lesions [2,5].
Rheumatoid nodules sometimes visible on gross examination of the heart have been described mainly after histological examination in patients who died of heart failure or pericardial involvement, and in those who died of conductive disorders. They consist of a central area of fibrinoid necrosis surrounded by a border of fibroblasts, macrophages, and connective tissue with a lymphoplasmacytic infiltrate, or even extensive fibrosis. Infiltration by these nodules of the nodal tissue, or His-Purkinje bundle by their location at the level of the interventricular septum, could be the cause of conductive disorders [2].
The location of these nodules determines cardiac symptomatology, so intramyocardial localization rarely affects cardiac function, however extensive localization of these nodules at the level of the myocardium has been associated with congestive heart failure and also with intracardiac abscesses due to superinfection of nodules leading to perforations [6].
The valvular location of nodules is also described in the literature, affecting in order of frequency most often the mitral valve, followed by the aortic valve, then tricuspid and finally the pulmonary valve. This localization of the nodules results in valve deformities or almost incompetence of the valve. An exceptional case of nodule necrosis in the aortic valve complicated by perforation of the Valsalva sinus thus producing a fistula with the VD has been described by Thery et al [6].
The rheumatoid cardiac nodules described in the literature are all infiltrated into the heart tissue. An exceptional case of rheumatoid nodule has been reported, described as a mobile mass of the left atrium that prolabades through the mitral valve, mimicking a myxoma. This observation is similar to our case in its description but with a straight location.
The toxicity of chloroquine treatment is well known. Its complications are mainly represented by neuromyopathy, corneal deposits and skin damage. On the other hand, cardiac toxicity is rare but serious, it can be responsible for congestive heart failure, restrictive cardiomyopathy and especially conduction disorders. Formal proof of this toxicity can be provided directly by endomyocardial biopsy by showing hypertrophy of the vacuole-filled myocytes, containing dense lamellar material and large lysosomes. On the other hand, the evidence may be indirect by noting the absence of an underlying pathology that could explain the conductive disorder. These complications occur in varying cumulative doses of 100 to 2500 g. [2]. Our patient did not have these doses, she only took 36 g of chloroquine in 6 months. In addition, other clinical signs of toxicity, cutaneous, ocular and neuromyopathy are absent in her.
Amyloid infiltration of conductive tissue has been reported by Thery et al.[7][13]; This appears to be the only documented case of cardiac amyloidosis secondary to rheumatoid arthritis that resulted in atrioventricular block. This involvement is rare, no case out of the 62 Lebowitz patients[8] had a major conductive disorder despite the presence of 10 cases of cardiac amyloidosis [2].
Rheumatoid vasculitis remains exceptional, accounting for only 1% of patients with rheumatoid arthritis[2].
The association of RA with an intracardiac mass has been described in the literature, among the main etiologies not noted: rheumatoid nodules, cancers, granulomatosis of the valve tissue, intracavitary thrombus and finally infective endocarditis.
Rheumatoid arthritis is known to be a disease, especially on methotrexate, promoting lymphomas and melanomas[9, 10]. The association between leiomyosarcoma, rheumatoid arthritis and methotrexate was already mentioned in 2001 by Hannequin et al Et Houitte et al in 2010 [11,12].
Paradoxical sarcoidosis-like reactions have been reported after treatment with anti-TumorNecrosis Factor α (anti-TNFα). Clinical presentations are varied, but most often involve typical mediastinal pulmonary involvement. More rarely, isolated granulomatous localizations have been described, which can affect different organs, posing a diagnostic problem.
A clinical case was reported in 2021 by Ngoufack et al [13] describing granulomatous cardiac valve localization complicating treatment with etanercept, in the form of a mobile mass on the mitral valve, in a 26-year-old patient with rheumatoid arthritis.
Patients with RA have a more than doubled risk of Venous Thromboembolic Disease (VTE). The prognosis for RA depends primarily on cardiovascular events, including venous thromboembolic disease.
The incidence rate of VTE in RA is estimated to be 4 cases per 1,000 patient-years [1].
The etiology of thrombotic tendency in RA is related to various mechanisms and causal factors (anti-phospholipid antibodies, hyperhomocysteinemia, inflammation, etc.): vascular injury, hypercoagulation and venous stasis, the three components of the Virchow triad, are activated in RA patients [1].
Similarly, observational studies indicate that uncontrolled RA—defined as the need to replace disease-modifying antirheumatic drug (DMARD)-type disease-modifying antirheumatic therapy—increases the incidence of VTE. In addition, DMARDs may have an influence on the risk of VTE, especially at the beginning of treatment. Several biologic DMARDs such as tofacitinib have been associated with an increased risk of VTE [1]. In our observation the finding of the mass was at the fifth month of treatment with leflunomide, suggests the possibility of a thrombus, however the non-regression of the mass under effective anticoagulation makes this proposal less likely.
The stimulation threshold can be modified in different situations, ranging from physiological states such as sleep and the postprandial period where the threshold increases, to pathological states such as ischemia, fibrosis, fluid electrolyte disorders such as dyskalemia, defibrillation, dysthroidism and finally drug intake [14].
Vaughan Williams' Class I antiarrhythmic drugs blocking sodium channels increase the threshold for pacemaker stimulation [15-17].
Amiodarone, a class III antiarrhythmic, is one of the antiarrhythmic drugs described in situations of increased stimulation threshold,[18] unlike the rest of the class III antiarrhythmics, this can probably be explained by the class I effect that amiodarone possesses[19].
On the basis of these findings, it can be deduced that any drug with a class I effect of antiarrhythmics is electrophysiologically likely to increase the stimulation threshold.
Thus, chloroquine, a molecule widely used in the treatment of RA, known for its property of blocking sodium and potassium channels such as hERG thus giving it an effect of class I and class III antiarrhythmics, is implicated according to cases in the literature in the increase of the stimulation threshold, this is probably explained by its blocking effect on sodium channels [14].
In our case, chloroquine was discontinued when the patient experienced syncope, but she was on methotrexate, with a weekly injection. The periodic onset of vertigo and syncope one day after the methotrexate injection suggests the possibility of an increase in the stimulation threshold, but we are not sure because the patient did not consult in time.
Methotrexate is known for its adverse effects and toxicity on many organs, however this molecule is not known for its cardiovascular effects [20]. Based on our literature review, no cases of increased stimulation threshold have been described. Our case is the first to implicate methotrexate in the possibility of increasing the stimulation threshold.
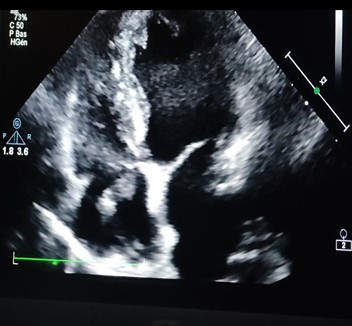
Figure 1: Echocardiography image: showing the mass in the atrium straight measuring 17x6 mm.
Conclusion
Cardiovascular complications during RA determine the prognosis of patients. Conductive disorders during the course of rheumatoid arthritis, although rare, should be systematically detected by regular cardiac work-up. Intracardiac masses always pose problems of etiological diagnosis, especially in the context of a systemic disease such as RA.
References
- Ketfi C, et al. Risk of venous thromboembolic disease in rheumatoid arthritis. Journal of Rheumatism, 2021; 88(5): pp. 338-345.
- Hamda K, et al. Rheumatoid nodule responsible for complete atrioventricular block: diagnosis by transesophageal echocardiography. Annals of Cardiology and Angeiology - ANN CARDIOL ANGEIOL, 2004; 53: pp. 101-104.
- Corrao S, et al. Heart involvement in Rheumatoid Arthritis: Systematic review and meta-analysis. International journal of cardiology, 2013; 167(5): p. 2031-2038.
- Ivy KN, et al. Rheumatoid Arthritis-Associated Valvulitis Or Post-Streptocococcal Rhematic Valve Disease: Can We Rely On Anitsckhow Cells? Journal of the American College of Cardiology, 2021; 77(18, Supplement 1): p. 2881.
- Tilstra J, Lienesch D. Rheumatoid Nodules. Dermatologic clinics, 2015; 33: p. 361-371.
- Webber MD, Selsky EJ, Roper PA. Identification of a mobile intracardiac rheumatoid nodule mimicking an atrial myxoma. Journal of the American Society of Echocardiography, 1995; 8(6): p. 961-964.
- Thery CL, et al. The atrioventricular block of rheumatoid arthritis: the histological study of the His-Tawara system (about a new observation) Lille M, 1977; 22: 92–97.
- WB L. The heart in rheumatoid arthritis –a clinical and pathological study of 62 cases. Ann Med Int, 1963; 58: 102–123.
- Buchbinder R, et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum, 2008; 59(6): p. 794-799.
- Franklin J, et al. Incidence of lymphoma in a large primary care derived cohort of inflammatory polyarthritis. Annals of the rheumatic diseases, 2006; 65: p. 617-622.
- Hannequin J, et al. Leiomyosarcoma, rheumatoid arthritis and methotrexate: an association? Joint, bone, spine: revue du rheumatisme, 2001; 68: p. 445.
- Houitte R, et al. Leiomyosarcoma of the left renal vein in the context of rheumatoid arthritis on methotrexate. J Mal Vasc, 2010; 35(3).
- Ngoufack C, et al. Mitral valve granulomatosis: a case of paradoxical reaction complicating etanercept treatment in rheumatoid arthritis. Journal of Rheumatism, 2021; 88(6): pp. 459-462.
- Huang PH, et al. Implanted pacemaker failure caused by the antirheumatic drug hydroxychloroquine: Lupus, 2003; 12(9): 725-727. doi: 10.1191/0961203303lu435xx.
- Kang T, et al. A Case of Acute Ventricular Capture Threshold Rise Associated with Flecainide Acetate. Yonsei medical journal, 2006; 47: p. 152-154.
- Fornieles-Pérez H, et al. Documentation of acute rise in ventricular capture thresholds associated with flecainide acetate. Pacing Clin Electrophysiol, 2002; 25(5): p. 871-872.
- Fearon I, Gautier M. Prolonged action potentials in cardiac Purkinje cells: A distinct phenotype arising from a distinct sodium channel. Experimental physiology, 2007; 92: p. 1-2.
- Kim J, et al. Increased threshold in nonselective His-bundle pacing suspected to be caused by amiodarone: HeartRhythm Case Rep, 2018; 5(2): 112-114. doi: 10.1016/j.hrcr.2018.11.007.
- Jiang M, et al. Increased capture threshold in permanent His-bundle pacing associated with flecainide. Pacing Clin Electrophysiol, 2020; 43(4): p. 360-363.
- Campbell JM, et al. Methotrexate-induced toxicity pharmacogenetics: an umbrella review of systematic reviews and meta-analyses. Cancer Chemother Pharmacol, 2016; 78(1): p. 27-39.

