Dependence of Ki-67 Values on Tumor Size in 1040 Breast Cancers of Five Immunohistochemical Phenotypes: An Argument for a Minority of Invasive Breast Cancers with High Mitotic Rates of a Little Dependence on ER and HER2 Expressions
Sven Kurbel1,2,* and Branko Dmitrovic3
1Josip Juraj Strossmayer University of Osijek, Medical Faculty, J Huttlera 4, Osijek 31000, Croatia
2Juraj Dobrila University of Pula, Medical Faculty, Zagrebačka 30, Pula 52100, Croatia
3Josip Juraj Strossmayer University of Osijek, Faculty of Dental, Medicine and Health, Crkvena 21, Osijek 31000, Croatia
Received Date: 09/10/2023; Published Date: 12/03/2024
*Corresponding author: Sven Kurbel, Josip Juraj Strossmayer University of Osijek, Medical Faculty, J Huttlera 4, Osijek 31000, Croatia; Juraj Dobrila University of Pula, Medical Faculty, Zagrebačka 30, Pula 52100, Croatia
Abstract
A single institution data set of 1040 breast cancer patients have been used to compare tumor diameter with the corresponding Ki-67 value.
Results; All ER positive patients, similar to their three HER2 subgroups show significant positive correlation between the tumor diameter and the corresponding Ki-67 values, suggesting that larger cancers show higher Ki67 values. ER negative breast cancers showed no correlation between their size and their Ki-67 value, regardless of the HER2 expression.
Conclusions: All isolated phenotypic clusters contained two EM subgroups per slope of mitotic activity the high and the low clusters, separated somewhere near the 18%/cm of tumor diameter threshold of Ki-67/cm. Tumors with higher levels of Ki-67 per unit diameter of tumors did not show high dependence on ER and HER2 expression. In pure ER positive and HER2-absent tumors, the most of them were with small and pred citable mitotic slope, due to dependence on ER-ligands coming by neo vasculature. It was the majority of ER and PgR negative tumors with a negative impact of HER2 expression, leading to more tumors of predictable lower Ki-67 values. In ER-positive and ER-negative tumors with higher ratios of Ki-67 values per unit diameter, the local growth stimulation was possibly mainly dependent on still undefined Para mediator which is in negative interaction with HER2 and with ER expressions.
Keywords: Invasive breast cancer; Breast cancer types; Immunohistochemistry; Ki-67 value
Introduction
Background:
When making clinical decisions regarding breast cancer (BC) patients, estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor 2 (HER2) and Ki-67 are important phenotype features [1, 2] that allow classification of breast tumors into five immunohistochemical phenotypes. Luminal A, Luminal B1 and Luminal B2 are breast cancer types with positive ER or PgR on tumor cells. Tumors lacking both ER and PgR are recognized as the triple-negative (HER2 expression ranges from absent (0+) to 2+) and the “pure HER2" overexpressing breast tumors (only HER2 3+).
At the St. Gallen 2013, it was concluded that: ". The majority of the Panel accepted that a useful surrogate definition of Luminal A-like as distinct from Luminal B-like disease could be made using a combination of ER, PgR and Ki-67, without requiring molecular diagnostics" [3]. After that, a question of the threshold above which a cancer should be considered more aggressive was opened for discussion. Although the 14% threshold in separating Luminal A and Luminal B1 breast cancers has already been used, at the same conference it was concluded that: ". The Panel noted that standardized cut-offs for Ki-67 have not been established and laboratory specific values should be used, but the majority of the Panel voted that a threshold of >20% was clearly indicative of ‘high' Ki-67 status" [3]. This means that a voting majority of the expert panel has defined a single Ki-67 threshold for all breast cancer patients, regardless of tumor type, tumor size or age. This threshold has not been tested in a randomized trial of a sufficient size prior to this decision. Possibly this was a proper procedure for choosing a cut-off value for an independent feature, but there are several references suggesting that the Ki-67 values in breast cancers are related to the size of breast tumor.
The question:
The usual division into 5 types of BC does not fully meet our clinical requirements, prognostic possibilities of the tumor classification within Luminal A, B1 or B2 breast tumors, HER2 overexpressed or the “triple negative” group remains of limited value when applied to an individual patient. The question is whether the intensity of mitotic activity in which the tumors were found by the surgery meets the input criteria? Therefore, the Ki-67 values were here converted into linear velocities as numbers expressed as changes in percentage of the Ki-67 value (Δ%) / cm of the tumor diameter (d). Two EM clusters were detected among 1040 patients operated from invasive breast cancers of known diameter and the Ki-67 value (Table 1).
Patients and Methods
We have used a single institution data set of breast cancer patients that has already been assembled as a part of a completed research project financed by the Croatian Ministry of Science (219–2192382-2426). Before the grant submission to the Croatian Ministry of Science and Education, collecting of breast cancer data was approved by the Ethical Committee of Osijek Medical Faculty, as compliant with the Helsinki Declaration. In this study, out of 1180 consecutive invasive ductal breast cancer patients diagnosed and treated in Osijek Clinical Hospital in Croatia, 1040 patients with measurable tumors up to the diameter of 5 cm were included. This consecutive series of patients has already been described in details in several published papers [4-6] and the data set has been published as supplementary data to one of these papers [6].
Statistical analysis
Collected data were organized in a spreadsheet by StatSoft, Inc. (2011) STATISTICA (data analysis softwaresystem), version 10. www.statsoft.com. Scatterplots and the EM clustering with nonparametric tests were used.
Results
Integration of the Ki-67 values and tumor diameter in a single continuous value
Each tumor is the result of cancer growth in individual patients and is determined at the time of surgery. If certain types of breast cancer have significantly predictable mitotic activity relative to tumor size, then these tumors in their growth depended on a shared, common mitotic mechanism that accelerated cell division under conditions determined by tumor size.
In order to better define the moment of surgery as the cessation of ever-growing tumor growth, we tried to calculate a value called "mitotic slope" and is equivalent to the rate of mitotic activity per centimeter of diameter and are calculated as the quotient Ki-67% and the tumor diameter.
Table 1 shows HER2 status (HER2 absent (0), HER2 present (HER2 1+ or 2+) and HER2 overexpressed (3+). These subgroups were chosen as compatible to the five breast cancer types, but also more distinctive in evaluating the HER2 presence in tumors that are not HER2 3+. As already reported for the same data set [6], the HER2 absent breast cancers might held some distinctions from other tumors.
As shown in Fig. 1: a scatterplot of 1040 Ki-67 values against the cancer diameter clearly shows that there is a significant linear correlation between these variables (Figure 1).
In search of the Ki-67 & breast cancer size relations
Several details should be noted in Table 1. All patients and also all ER positive patients, similar to their three HER2 subgroups show significant positive correlation between the tumor diameter and the corresponding Ki-67 values, suggesting that larger cancers show higher Ki67 values. ER negative breast cancers showed no correlation between their size and their Ki-67 value, regardless of the HER2 expression.
Figure 2. shows relations between Ki-67 values and breast cancer diameters for according to their immunohistochemical (IHC) phenotypes. Figure 3. shows relations between Ki-67 values and six breast cancer possible variants of their IHC phenotypes. Dispersion of scatter plots for the ER negative tumors allowed no significant correlations (Figure 2 and 3).
These observations suggest that ER positive tumors share biological features that make their growth speed and mitotic activity so uniform that we might use relations in invasive breast tumors in Table 1 to calculate the expected Ki-67 value for an ER positive breast cancer of a certain size. In ER negative tumors, mitotic activities seem unrelated to their size.
Table 1: Six possible IHC cancer phenotypes found among 1040 invasive BC patients.

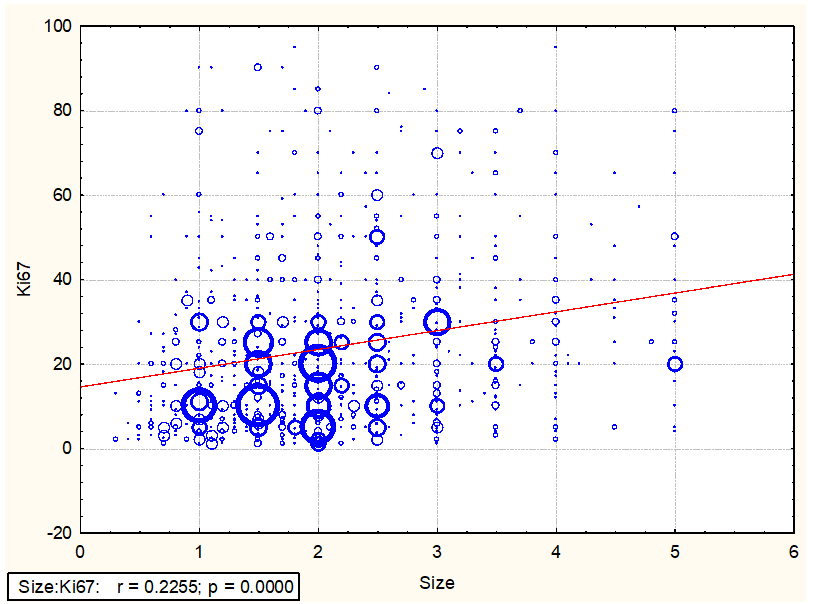
Figure 1: Relations between Ki-67 values and the tumor diameter (in centimeters) of 1040 cases of invasive breast cancers.
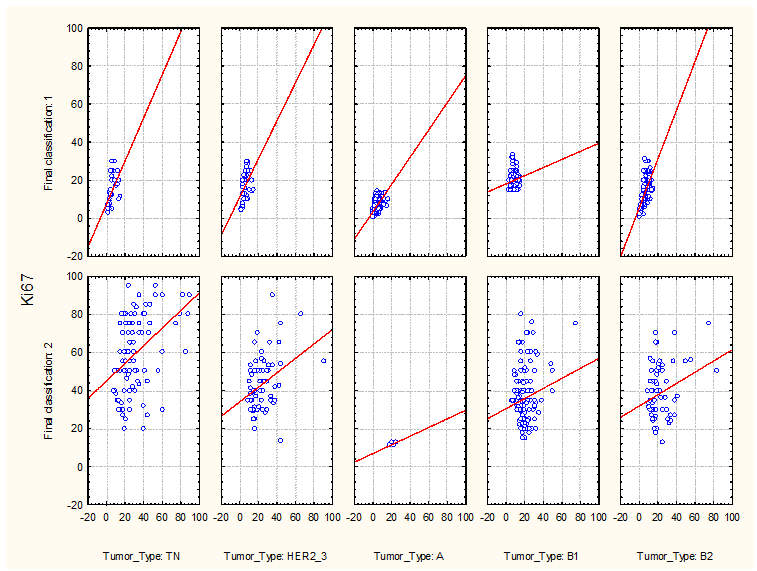
Figure 2: Relations between Ki-67 values and breast cancer diameters for according to their immunohistochemical (IHC) phenotypes.

Figure 3: Relations between Ki-67 values and six breast cancer possible variants of their IHC phenotypes.
Discussion
An intriguing relation between the tumor size and Ki-67 values was found only in our patients with ER positive cancers. Both pure HER2 overexpressed and triple negative cancers have showed large dispersions of Ki67 values, thus the Ki67 values seem unrelated to their tumor size.
These observations require validation in unrelated data sets, but, if proved, they might suggest that there are separate roads to the occurrence and growth of breast cancers od different types, and the one driven by estrogens is the most predictable in cancers that are ER+/HER2 0.
This is in concordance with our previous and unrelated statistical approach to the same data set [6], that also suggested that HER2 absent cancers might be distinguishable both among Luminal A&B1 tumors and among triple negative breast cancers. In here presented analysis, ER negative and also HER2 0 cancers showed only a weak, unsignificant relation with the tumor size, although more evident than the remaining ER negative cancers.
An intriguing question is whether ER positive tumors are larger due to high mitotic activities or are mitotic activities increased due to larger tumors?
- The former suggests that the mitotic activity in ER positive breast cancers is defined at the time of occurrence and remains almost unaltered during growth. Tumors with higher mitotic activities grow faster and we get a significant correlation between Ki-67 values and tumor size in consecutive cases.
- The latter interpretation suggests that all ER positive tumors start slowly with low Ki-67 values and increase their Ki-67 values when become larger. Since these tumors are estrogen dependent and their relation between Ki-67 and tumor size was present similarly in premenopausal and postmenopausal patients, increased mitotic activities in larger tumors might result from paracrine mediators in the HER2 route etc.
Another mechanism that might be more present in larger tumors is the self-seeding of metastatic cells that return to the primary site and start new centers of mitotic activities [7]. In planning tests of our treatment strategies, the latter interpretation might offer more possibilities than the former interpretation, based on idea that the mitotic rate was determined at the very early phase of cancer development.
Ki-67 as a common cell proliferation marker is frequently determined in routine clinical work and Ki-67 expression is associated with common histopathological parameters, but is an additional independent prognostic parameter for DFS and OS in breast cancer patients enlisted in the Bavarian Cancer Registry in Germany [8].
I have also been reported important in other settings [9,10], including the prostate cancer [11].
Breast cancer is the most common cancer in females [10], and appropriate biomarkers play significant role in predicting the prognosis and decide the specific therapy to each patient. Among 92 patients, Ki-67 expression in estrogen and progesterone receptor positive tumors showed lower values than estrogen and progesterone negative tumors, while higher Ki-67 expression was more frequently associated with HER2-positive.
Conclusions
All isolated phenotypic clusters contained two EM subgroups per slope of mitotic activity the high and the low clusters, separated somewhere near the 18%/cm of tumor diameter treshold of %ki-67/cm.
Tumors with higher levels of Ki-67 per unit diameter of tumors did not show high dependence on ER and HER2 expression.
In pure ER positive and HER2-absent tumors, the most of them were with small and predictable mitotic slope, due to dependence on ER-ligands coming by neovasculature.
It was the maiority of ER and PgR negative tumors wirth a negative impact of HER2 expression, leading to more tumors of predictable lower Ki-67 values.
In ER-positive and ER-negative tumors with higher ratios of Ki-67 values per unit diameter, the local growth stimulation was possibly mainly dependent on still undefined paramediator which is in negative interaction with HER2 and with ER expressions.
References
- Yamamoto-Ibusuki M, Yamamoto Y, Yamamoto S, Fujiwara S, Fu P, Honda Y, et al. Comparison of prognostic values between combined immunohistochemical score of estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, Ki-67 and the corresponding gene expression score in breast cancer. Mod Pathol, 2013; 26(1): 79–86. doi:10.1038/modpathol.2012.151.
- Inwald EC, Koller M, Klinkhammer-Schalke M, Zeman F, Hofstädter F, Gerstenhauer M, et al. 4-IHC classification of breast cancer subtypes in a large cohort of a clinical cancer registry: use in clinical routine for therapeutic decisions and its effect on survival. Breast Cancer Res Treat, 2015; 153(3): 647–658. doi:10.1007/s10549-015-3572-3.
- Shaaban AM, Sloane JP, West CR, Foster CS. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. Am J Pathol, 2002; 160(2): 597-604. doi: 10.1016/s0002-9440(10)64879-1.
- Kurbel S. In search of triple-negative DCIS: tumor-type dependent model of breast cancer progression from DCIS to the invasive cancer. Tumour Biol, 2013; 34(1): 1-7. doi: 10.1007/s13277-012-0602-1.
- Kurbel S. Model of tumor-associated epigenetic changes of HER2, ER, and PgR expression in invasive breast cancer phenotypes. Tumour Biol, 2013; 34(4): 2011-2017. doi: 10.1007/s13277-013-0809-9.
- Kurbel S, Dmitrovi B, Marjanoviæ K, Vrbanec D, Jureti A. Distribution of Ki-67 values within HER2 & ER/PgR expression variants of ductal breast cancers as a potential link between IHC features and breast cancer biology.BMC Cancer, 2017; 17(1): 231. doi: 10.1186/s12885-017-3212-x.
- Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell, 2009; 139(7): 1315-1326. doi: 10.1016/j.cell.2009.11.025.
- Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat, 2013; 139(2): 539-552. doi: 10.1007/s10549-013-2560-8
- Zhu X, Chen L, Huang B, Wang Y, Ji L, Wu J, et al. The prognostic and predictive potential of Ki-67 in triple-negative breast cancer. Sci Rep, 2020; 10(1): 225. doi: 10.1038/s41598-019-57094-3
- Ragab HM, Samy N, Afify M, El Maksoud NA, Shaaban HM. Assessment of Ki-67 as a potential biomarker in patients with breast cancer. J Genet Eng Biotechnol, 2018; 16(2): 479-484. doi: 10.1016/j.jgeb.2018.03.002.
- Fisher G, Yang Z, Kudahetti S, et al. Prognostic value of Ki-67 for prostate cancer death in a conservatively managed cohort. Br J Cancer, 2013; 108: 271–277. doi: 10.1038/bjc.2012.598

