Small Bowel Leiomyosarcoma Receiving Complied with Intestinal Occlusion
Bouali Mounir, Eddaoudi Yassine, El Wassi Anas*, El Bakouri Abdelilah, El Hattabi Khalid, Bensardi Fatimazahra and Fadil Abdelaziz
Visceral Surgery Emergency Department P35, University Hospital Center Ibn Rochd, Morocco
Received Date: 20/08/2023; Published Date: 11/01/2024
*Corresponding author: El Wassi Anas, Visceral Surgery Emergency Department P35, University Hospital Center Ibn Rochd, Casablanca, Morocco
Abstract
Introduction: Leiomyosarcoma accounts for approximately 20% of adult small bowel malignancies. Indeed, only 46 cases have been reported in the literature. This rarity explains the lack of information concerning the diagnosis, treatment and prognosis of this condition. The authors report a case of recurrent leiomyosarcoma, specifying its clinical, radiological and histopathological characteristics and discuss the diagnostic and therapeutic difficulties.
Case Presentation: We report a case we report the case of a patient admitted for acute intestinal obstruction on small bowel leiomyosarcoma in the department of Emergency visceral surgery.
Our patient as antecedent: operated for leiomyosarcoma of the broad ligament having benefited from a hysterectomy without adnexal conservation in June 2021, was admitted in our service P35 for occlusive syndrome made stop of matter and gas with pelvic pains. Clinical examination revealed a patient in poor general condition, abdominal palpation found a large pelvic mass and rectal touch: empty rectal ampulla, Radiology of the abdomen without preparation found several hydroaeric levels in the bowel.
The patient underwent an abdominal CT scan which showed: five abdominopelvic masses with a voluminous pelvic mass enclosing the ileal intestines responsible for significant distension of the upstream intestines associated with a moderate amount of peritoneal effusion and a multi-nodular liver.
At the surgical exploration we noted the presence of a medium abundance ascites with clear yellow liquid, the presence of several tumor-like masses, the first one located at 40 cm from the ICJ measuring 13 cm in diameter realizing a stenosing magma of coves with distension in front of 5 cm , the second adherent to the sigmoid and the third at the pelvic level, each measuring 10 cm in diameter and another measuring 2 cm at the subumbilical level, with a liver full of metastases.
In view of this result, a decision was made to perform a 50 cm graft resection with a stenosing loop magma adherent to a necrotic tumor-like mass and an ileostomy at 30 cm from the IJC with a biopsy of a hepatic metastasis at the level of segment IV and an exeresis biopsy of a subumbilical mass of 2 cm in diameter.
Clinical Discussion: Primary leiomyosarcoma of the small intestine is very rare. It differs in all respects from that of adults to that of children, these tumors are often revealed by acute complications.
Leiomyosarcoma remains relatively confined to the organ and is often small in size at the time of diagnosis. A palpable abdominal mass is found in only 40% of cases. This tumor is often located in the proximal groin (75%).
Surgical excision is the treatment for this condition. Moreover, the resectability rate in children is much higher than in adults.
The standard histological examination allows the diagnosis in typical cases where cytonuclear abnormalities and mitoses are clear.
Conclusion: Small bowel leiomyosarcoma is very rare. Its prognosis is slightly better in adults than in children, perhaps because of the early clinical presentation in children The treatment remains surgical; adjuvant chemotherapy and radiotherapy could improve the reserved prognosis of this tumor by reducing local recurrences without changing the survival and chemotherapy has not been proven to be effective.
Preoperative diagnosis is rarely made; leiomyosarcoma most often presents as a trivial myoma or necrobiosis.
Keywords: Leiomyosarcoma; Small bowel tumour; Acute intestinal obstruction
Introduction
Leiomyosarcoma accounts for approximately 20% of adult small bowel malignancies. [1,2]. Indeed, only 46 cases have been reported in the literature [3]. This rarity explains the lack of information concerning the diagnosis, treatment and prognosis of this condition.
Uterine sarcomas are rare uterine cancers and represent between 2 and 6% of malignant tumors of the body of the uterus [1,2]. These tumors have a poor prognosis and are characterized by a high degree of anatomical heterogeneity. Although it is accepted that the reference treatment of uterine sarcomas is surgical, the place of adjuvant treatments remains debated [1,3,4]. The authors report a case of recurrent leiomyosarcoma, specifying its clinical, radiological and histopathological characteristics and discuss the diagnostic and therapeutic difficulties.
Patient Et Observation
Mrs BN, 46 years old, 2nd gesture, 2nd pare, having as antecedent: operated for leiomyosarcoma of the broad ligament having benefited from a hysterectomy without adnexal conservation in June 2021, of low socio-economic level, was admitted in our service for occlusive syndrome made stop of matter and gas with pelvic pains. Clinical examination revealed a patient in poor general condition, abdominal palpation found a large pelvic mass and rectal touch: empty rectal ampulla, the rest of the general examination was unremarkable. Radiology of the abdomen without preparation found several hydroaeric levels in the bowel. The blood count showed a microcytic hypochromic anemia at 8.6/dl. The rest of the preoperative workup was without abnormalities.
The patient underwent an abdominal CT scan which showed: Presence of 5 abdornino-pelvic tissue masses with extensive necrosis: One in the left flank 10 cm, one in the left iliac fossa in 11 cm, one umbilical subcutaneous at 1.5 cm, one right iliac 7 cm and one pelvic 9 cm. The large pelvic mass encompasses the pelvic ileal intestines with significant hydro-aerosic dilatation upstream and flattening of the downstream digestive intestines with moderate peritoneal effusion in the inter-intestines. The liver of normal volume is the site of multiple hypodense nodules, the largest of which is located in segment IV and measures 4.5 cm in long axis.
In total: CT scan of five abdominopelvic masses with a voluminous pelvic mass enclosing the ileal intestines responsible for significant distension of the upstream intestines associated with a moderate amount of peritoneal effusion and a multi-nodular liver (Figure 1).
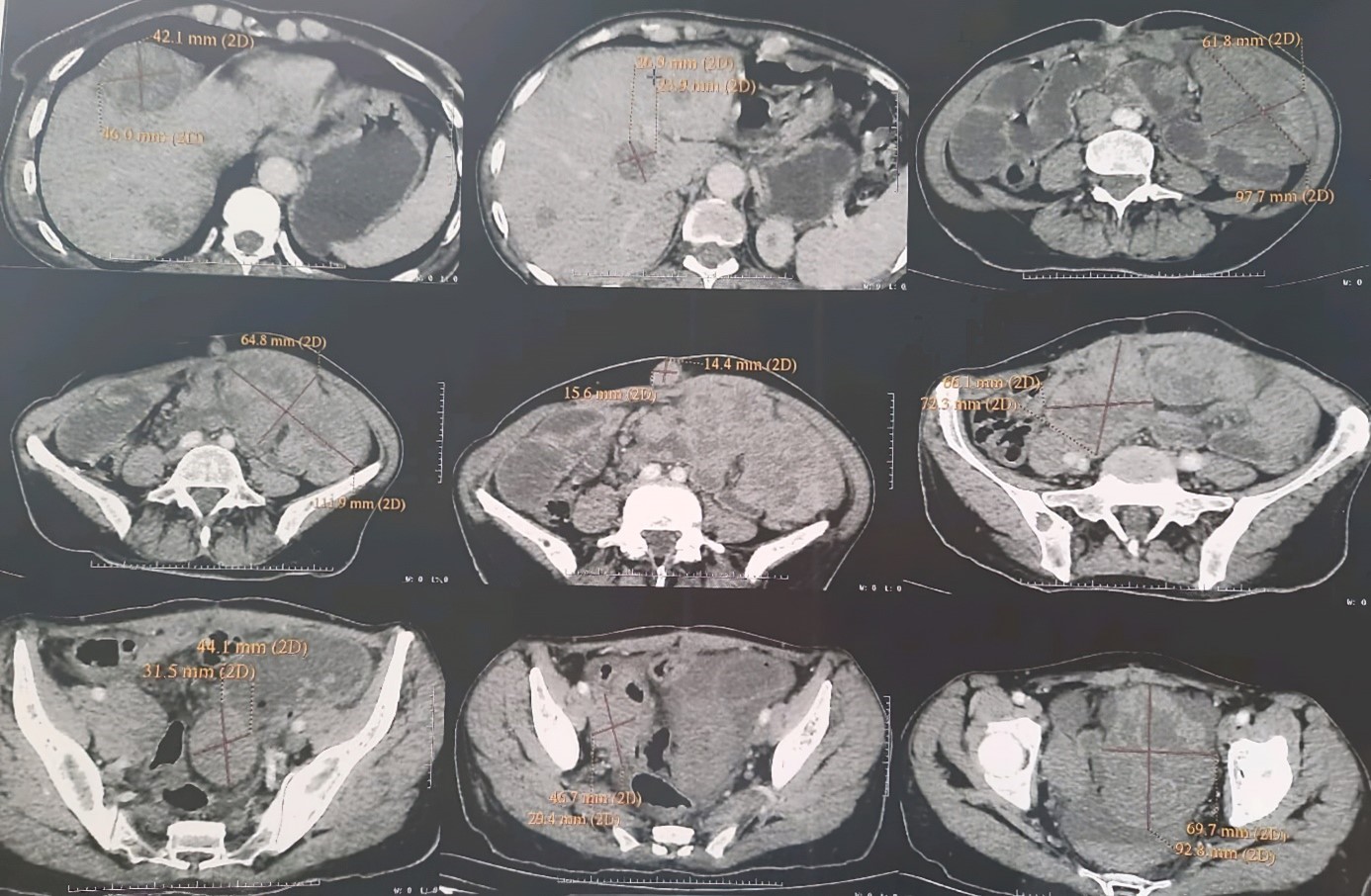
Figure 1: Abdominal-pelvic CT.
At the surgical exploration we noted the presence of a medium abundance ascites with clear yellow liquid, the presence of several tumor-like masses, the first one located at 40 cm from the ICJ measuring 13 cm in diameter realizing a stenosing magma of coves with distension in front of 5 cm (Figure 2), the second adherent to the sigmoid and the third at the pelvic level, each measuring 10 cm in diameter (Figure 3), and another measuring 2 cm at the subumbilical level (Figure 4), with a liver full of metastases.
the decision was to respect the mass causing the occlusion and to reduce the size of the accessible masses and to refer the patient for chemotherapy after the results of the anatomopathology
In view of this result, a decision was made to perform a 50 cm graft resection with a stenosing loop magma adherent to a necrotic tumor-like mass and an ileostomy at 30 cm from the IJC with a biopsy of a hepatic metastasis at the level of segment IV and an exeresis biopsy of a subumbilical mass of 2 cm in diameter.
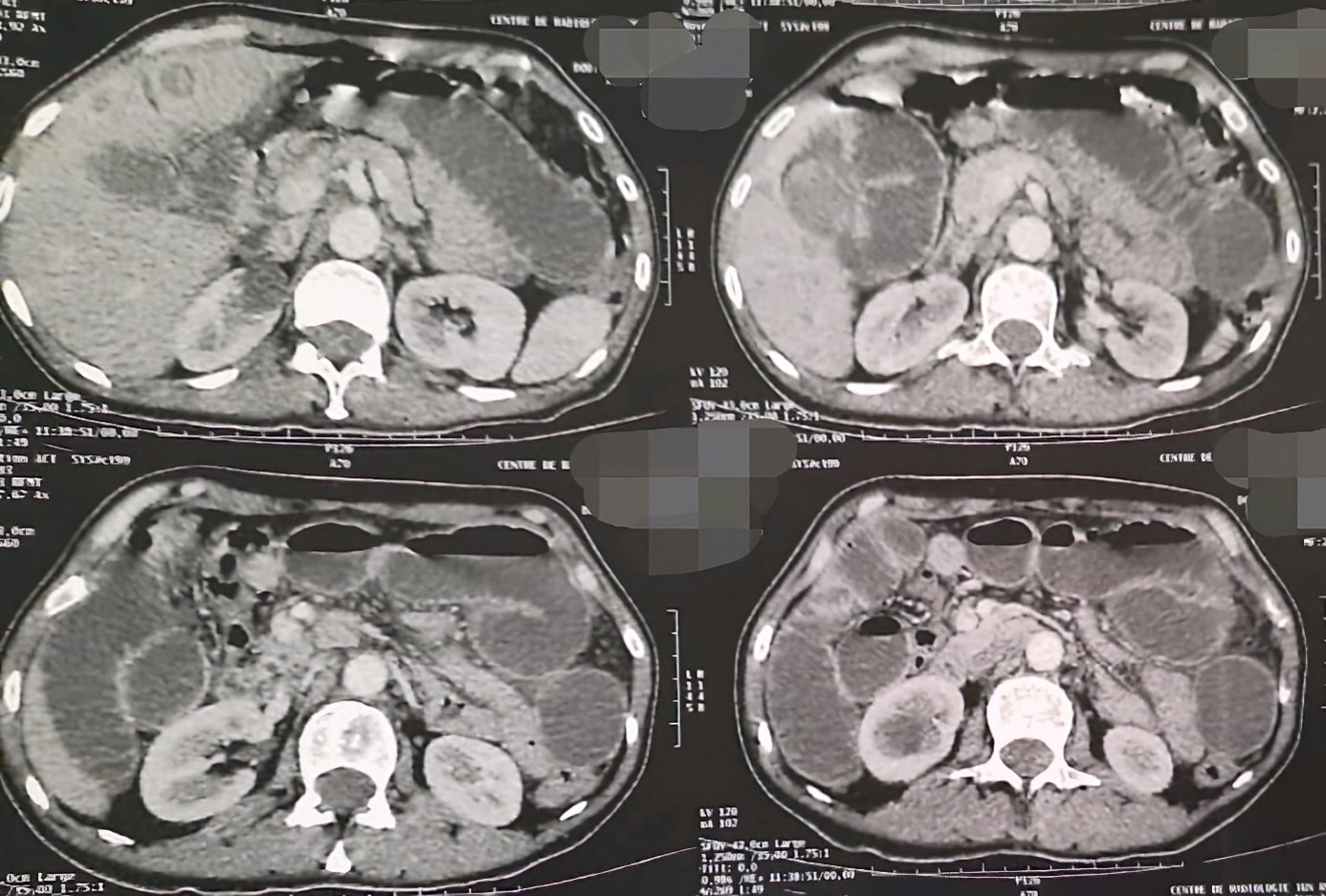
Figure 2: Intraoperative view showing: a magma of stenosing coves.

Figure 3: Intraoperative view showing.
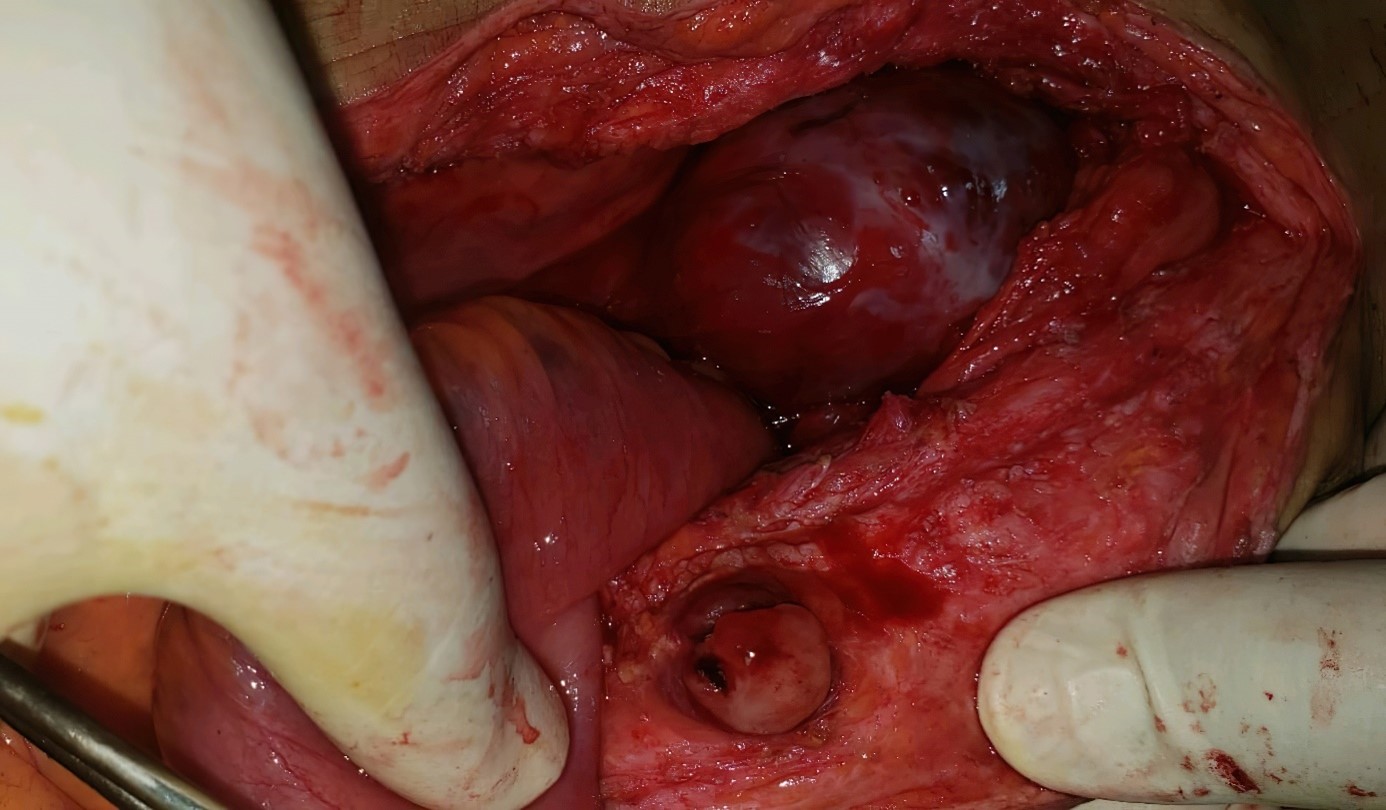
Figure 4: Intraoperative view showing.
The anathomathology results showed:A bowel resection specimen measuring 56 cm in length. The diameter varies between 3 and 4 cm.
The surface is congestive, with several nodular formations measuring between 10 cm and 2 cm in long axis. They remain 4.5 cm from one boundary and 5.5 cm from another boundary and do not infiltrate the bowel wall.
The tumor was classified as T4N1M1.
On section, they have a whitish fasciculated appearance and are the site of necrotic-hemorrhagic and myxoid reorganizations in some places, with the conclusion that they are a localization of a leiomyosarcoma in the bowel. With healthy resection limits with the same histological type in the subumbilical mass and absence of tumor cells in the liver biopsy.
The postoperative follow-up was marked by the resumption of transit on the 4th postoperative day with a soft abdomen and a clean dressing after the discharge on the 8th day.
The patient has been lost from sight since her discharge.
The patient had surgery for leiomyosarcoma of the broad ligament of the uterus and underwent a hysterectomy in June 2021 in a private facility and was admitted to us in October 2022 for the initial tumor stage established in 2021 without documentation of the circumstances of the first procedure or the conditions.
Regarding the histological variant of leiomyosarcoma, the anapath result showed a myxoid variant.
Discussion
Primary leiomyosarcoma of the small intestine is very rare [4-6]. It differs in all respects from that of adults to that of children [4]. These tumors are often revealed by acute complications: occlusion (59%), perforation (23%) [4] and rarely isolated lower bleeding [7].
Leiomyosarcoma remains relatively confined to the organ and is often small in size at the time of diagnosis [9, 10]. A palpable abdominal mass is found in only 40% of cases. This tumor is often located in the proximal groin (75%).
Surgical excision is the treatment for this condition [11,12]. Moreover, the resectability rate in children is much higher than in adults [6,13]. This surgery requires a large resection, sometimes extending to the surrounding organs, and lymph node dissection. Such a resection was possible in our patient and the histological study of the surgical specimen confirmed the absence of tumor invasion on the slices of the resection limits and of the nodes removed.
The standard histological examination allows the diagnosis in typical cases where cytonuclear abnormalities and mitoses are clear. Sometimes the differential diagnosis is difficult with benign muscular tumors of the digestive tract (leiomyomas) [14].
However, the use of immunohistochemistry helps in the diagnosis. The positivity of the anti-smooth muscle antibody (anti-actin antibody) of this tumor makes the diagnosis of a smooth muscle tumor and eliminates other gastrointestinal sarcomas [15].
The role of adjuvant chemotherapy is not clear [4], the results reported by Faldella et al [5], Angerpointuer et al [10], Chiotasso and Fazio [16] are disappointing and are consistent with the failure of chemotherapy. Mc Grath et al [2] report a complete clinical remission of 7 years, obtained with adjuvant chemo-radiotherapy after incomplete surgical resection.
Delayed diagnosis and lymph node involvement are elements of poor prognosis reported by most authors [5,
17-21].
Uterine leiomyosarcoma is a rare malignant tumor of a connective nature, developed at the expense of mesenchymal elements of the myometrium [5]. The relative frequency is 1.3% of all uterine cancers [6] and corresponds to 40 to 50% of uterine sarcomas [6,7]. They are characterized by a great heterogeneity on the anatomopathological level. These tumors have a poor prognosis, with a five-year survival rate of approximately 30% [1-8]. Their diagnosis must be made early, as the survival of patients is correlated with the tumor stage [1]. The average age of onset ranges from 45 to 55 years [9]. The disease most often occurs in patients who have been postmenopausal for 6-7 years. The clinical picture is not very specific. The three most frequent signs found in the literature are: genital hemorrhage, pelvic pain and pelvic mass [5,7,9]. Preoperative diagnosis is rarely made, as leiomyosarcoma most often presents as a trivial myoma or necrobiosis. The diagnosis is most often made on a hysterectomy specimen performed for uterine fibroid. This is the case of our patient. Classically, the rapid increase in volume of a leiomyoma and its softening should make the diagnosis suspicious [6]. Rapid enlargement of a leiomyoma is only found to be a leiomyosarcoma in 0.27% of cases. Ultrasound is not very specific [6]; leiomyosarcoma is most often limited to a single mass, there is no preferential location, and the base is large or pedunculated. A rapid increase in fibroid size and a necrobiose myoma image can be misleading. Several studies have analyzed the contribution of ultrasound and color Doppler and have not found any characteristics that would allow differentiation between a fibroid and a uterine sarcoma. Other authors compared indexes and color Doppler and found no morphological differences between fibroids, leiomyosarcomas and carcinosarcomas or differences in Doppler indexes between fibroids and leiomyosarcomas. Hysterosalpingography may show an enlarged uterine cavity with a polycyclic lacuna with clear contours and intra-lesional indentations. On CT scan [10], the lesions present as large areas of necrosis or cystic transformation, although non-specific, should raise the diagnosis.
CT scans can also be used to look for metastases in the lung, mesentery, omentum, retroperitoneal nodes, and spleen. These metastatic lesions often have a necrotic center. On MRI, the lesions show a heterogeneous signal in T2 weighting with hypersignal areas. On T1 weighting, they are iso- or hyposignal compared to the myometrial signal. They contrast intensely at the arterial time of injection; this contrast is often heterogeneous. At a later time, it is possible to assess whether there is tumor necrosis. [10]
MRI is the best tool for assessing local and locoregional extension. From an anatomopathological point of view, the appearance suggestive of a leiomyosarcoma is the soft, friable consistency, the white color, the size, which is frequently large (on average 10 cm in diameter), the site of necrotic and haemorrhagic changes, sometimes with clear invasion of the myometrium and the peritoneal serosa. The histological criteria used by most authors [5-7] for the diagnosis of leiomyosarcoma are those of Hendrickson and Kempson, adopted by Zaloudek and Norris. The diagnosis is made when there are more than 10 mitoses per field at objective 10 or if there are between 5 and 9 mitoses per field at objective 10 associated with cellular atypia or metastasis. Silverberg instituted a histologic grading system from well-differentiated to undifferentiated tumors, but this classification has limited prognostic value. Immunostaining with anti-desmin, anti-vimentin, and anti-1-anti-trypsin are useful to confirm the smooth muscle character of the proliferation. Extension [11] occurs by contiguity to the vagina, pelvis and abdomen. Metastases occur in order of frequency in the lung, liver, bone and brain. Lymphatic extension [11] is to the pelvic, para-aortic, mesenteric, mediastinal, hiliary and supra-clavicular nodes. The dominant prognostic factor is the mitotic activity of the tumor [5,12]; the higher the mitotic activity, the worse the prognosis. The stage of the disease clearly influences the course of the cancer [12]. The absence of necrosis and peritumoral hyalinization are good prognostic factors. Young age and premenopausal age are factors of good prognosis [5,7,12].
All stages combined, the 5-year survival is estimated to be between 25 and 40% [13]. The presence of metastases clearly influences survival. Indeed, the average survival is 19 months when there is a pulmonary metastasis and 12 months when several metastases are associated. Death occurs on average 8 months after the first recurrence. The rate of recurrence of leiomyosarcoma varies from 35 to 70% according to the authors, most often affecting the pelvis. They occur most often within two years of diagnosis, as in the case of our patient. Treatment is essentially surgical and must be complete from the start [9,12]. The first stage of the operation consists of a peritoneal cytology and an exploration of the abdomen. Most authors perform a non-conservative hysterectomy, although some have shown that conservation of the ovaries does not affect survival. Additional procedures depend on the exploration: visceral excision depending on the extension and pelvic curage if adenopathies are palpated. Radiotherapy reduces the incidence of pelvic recurrence but does not improve overall survival [9,13,14]: its place should be discussed on a case-by-case basis.
Chemotherapy may be proposed before surgery if the tumor is judged to be unresectable at the outset, or after surgery if tumor resection was not optimal, or in the case of distant metastases. The main protocols contain cis-platinum, adriamycin and ifosfamide and recently gemcitabine and docetaxel [13]. The efficacy of chemotherapy in this type of cancer is not yet spectacular, but new molecules, particularly in targeted therapy, are being investigated. Our patient was referred to the oncology department for further treatment and is now undergoing multidrug therapy with gemcitabine and docetaxel. When a leiomyosarcoma is discovered histologically on a hysterectomy specimen, no adjuvant treatment is required and in particular it is not necessary to reintervene for lymph node staging, as leiomyosarcoma is not very lymphophilic. However, it is preferable to perform an extension workup to ensure that there are no pulmonary or abdominal metastases (chest X-ray and abdomino-pelvic CT scan). Depending on the histological risk of uterine invasion, postoperative radiotherapy may be proposed.
Conclusion
Small bowel leiomyosarcoma is very rare. Its prognosis is slightly better in adults than in children, perhaps because of the early clinical presentation in children. The histological diagnosis is sometimes difficult; however, the presence of cytonuclear abnormalities and mitoses confirms malignancy. The main prognostic factor is mitotic activity. The smooth muscle origin of this tumor.
The smooth muscle origin of this tumor sometimes requires the use of immunohistochemistry.
The treatment remains surgical; adjuvant chemotherapy and radiotherapy could improve the reserved prognosis of this tumor by reducing local recurrences without changing the survival and chemotherapy has not been proven to be effective.
Preoperative diagnosis is rarely made; leiomyosarcoma most often presents as a trivial myoma or necrobiosis.
The consent statement: Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References
- Salazar OM, Bonfiglio TA, Patten SF, Keller BE, et al. Uterine Sarcomas: natural history, treatment and prognosis. Cancer, 1978; 42(3): 1152-1160.
- Livi L, Paiar F, Shah N, Blake P, et al. Uterine sarcoma: twenty- seven years of experience. Int J Radiat Oncol Biol Phys, 2003; 57(5): 1366-1373.
- Acharya S, Hensley ML, Montag AC, Fleming GF. Rare uterine Lancet Oncol, 2005; 6(12): 961-971.
- Livi L, Andreopoulou E, Shah N, Paiar F, et al. Treatment of uterine sarcoma at the Royal Marsden Hospital from 1974 to Clin Oncol (R Coll Radiol), 2004; 16(4): 261- 268.
- Van Dinh T, Woodruff JD. Leiomyosarcoma of the uterus. Am J Obstet Gynecol, 1982; 144(7): 817-823.
- Schwartz LB, Diamond MP, Schwartz PE. Leiomyosarcoma: clinical presentation. Am J Obstet Gynecol, 1993; 168(1 Pt 1): 180-183.
- Leibsohn S, Ablaing G, Mishell DR Jr, Schlaerth Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol, 1990; 162(4): 968-974 discussion 974-976.
- Nordal RR, Thoresen SO. Uterine sarcomas in Norway 1956- 1992: incidence, survival and mortality. Eur J Cancer, 1997; 33(6): 907-911.
- Socolov RV, Pricop FZ, Stratan D. La malignité du sarcome utérin, par comparaison avec les léiomyomes suspects : étude anatomo-clinique sur 16 Rev Fr Gynecol Obstet, 1992; 87(10): 457-460.
- Hata K, Hata T, Makihara K, Aoki S, et Sonographics findings of uterine leiomyosarcoma. Gynecol Obstet Invest, 1990; 30(4): 242-245.
- Goff BA, Rice LW, Fleischhacker D, Muntz HG, et al. Uterine leiomyosarcoma and endometrial stroma sarcoma: lymph node metastases and sites of recurence. Gynecol Oncol, 1993; 50(1): 105-109.
- Gerst PH, Levy J, Swaminathan K, Kshettry V, Albu Metastatic leiomyosarcoma of the uterus: anusual presentation of a case with late endobronchial and small bowel metastases. Gynecol Oncol, 1993; 49(2): 271-275.
- Echt G, Jepson J, Steel J, Langholz B, et al. Treatment of uterine Cancer. 1990; 66(1): 35-39.
- Major FJ, Blessing JA, Silverberg SG, Morrow CP, et Prognostic factors in early-stage uterine sarcoma; A Gynecologic Oncology Group study. Cancer, 1993; 71(4 Suppl): 1702-1709.
- Lauwers GY, Erlandson RA, Casper ES, Brennan MF, Woodruff JM. Gastrointestinal autonomic nerve tumors. A clinicopathological, immunohistochemical, and ultrastructural study of 12 cases. Am J Surg Pathol, 1993; 17: 887–897.
- Chiotzsso PJP, Fuio VW. Pronostic factors of 28 leiomyosarcomas of the small intestine. Surg Gynccol Obstet, 1982; 155: 197-202.
- El Shafie M, Spitz L, ikeda S. MrJilmant tumors of the small bowel in neonates presenting with perforation. J Pedlatr Surg, 1971; 6: 62-64
- McGrath P, Neifeld JP, Lawrence W, DeMay RM, Kay S, Horsley JS 3rd, et al. Improved survival following complete excision of retroperitoneal sarcomas. Ann Surg, 1984; 200: 200-204.
- Roth D, Farinacci CJ. Jejunal leiomyosarcoma in a newborn. Cancer, 1950; 3: 1039-1043.
- Solornons NW, Wagonfeld JB, Thomson S, et al. Leiomyosarcoma of the duodenum in a 10-year-old boy. Pediatrics, 976; 58: 268-273.
- Skandalkis JE, Gray SW, Schepard D. Smooth muscle tumors of the small intestine. Am J Gastroenterol, 1964; 42: 172-188.

