Oxaliplatin Induced Crystalline Retinopathy
Farhad Shahi1, Ali Makateb2,*, Hassan Khojasteh2 and Fatemeh Bazvand2
1Cancer Institute, Imam Khomeini Hospita , Tehran University of Medical Sciences, Iran
2Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Iran
Received Date: 20/08/2023; Published Date: 09/01/2024
*Corresponding author: Ali Makateb MD, Eye Research Center, Farabi Eye Hospital, Qazvin Square, Tehran, Iran
Abstract
We report a case of 72-year-old lady, known case of colorectal cancer under treatment with Oxaliplatin for maintenance therapy. She was treated by Oxaliplatin 130mg/m2 and Capecitabine 1gr/m2 bid for two weeks (famous Capox regimen) last year. She was referred to our clinic with chief complaint of ocular irritation, foreign body sensation and visual loss in her right eye. Following visual impairment in examination mainly in right eye and observation of scattered iridescent dots through retina in fundoscopy, Electroretinogram (ERG), and Optical Coherence Tomography (OCT) were done for her due to the suspicion of 2 differential diagnosis (toxic retinopathy and cancer associated retinopathy). Following paraclinic tests moderate to severe impairment of scotopic response and moderate impairment of photopic ERG test results and outer retina and RPE atrophy in OCT of both eyes were detected. Ocular toxicity has been mentioned as a rare and mainly reversible side effect of Oxaliplatin. In follow up exam 3 months later, imagings were performed again and the diagnosis of Oxaliplatin retinopathy was proposed due the results of paraclinic tests. This case is the first report of retinal toxicity (crystalline retinopathy due to Oxaliplatin) demonstrating iridescent dots through retina and permanent damage of outer retina and RPE layers. With increasing use of oxaliplatin in chemotherapeutic regimens in colorectal patients we should be aware of this possible side effect.
Keywords: Oxaliplatin; Electrooculography; Crystalline Retinopathy; Retinal pigment epithelium; Electroretinography
Introduction
Oxaliplatin is a third-generation platinum based chemotherapeutic agent usually used in a famous combination regimen of Folinic Acid (Leucovorin), 5-FU and Oxaliplatin (FOLFOX) given on a fortnightly schedule in colorectal and pancreatic cancer patients [1].
Similar to other chemotherapy agents, Oxaliplatin has also been associated with several side effects and almost all of them are associated with neurotoxicity [2]. Symptoms are usually reversible, but may persist for a longer period in spite of treatment cessation [2,3]. Hematological side effects, gastrointestinal complications and mild to severe hypersensitivity reactions such as anaphylaxis have also been reported [3–5].
Ocular toxicity is a rare side effect of Oxaliplatin. It can produce a variety of ocular symptoms, which are mostly transient and reversible [6]. Adverse symptoms vary from mild changes, such as dry eye and conjunctivitis to more severe and permanent changes, such as visual field and retinal damages [6]. Patients on Oxaliplatin therapy regimens may complain of decreased visual acuity, contracted visual field, change in color vision and other symptoms. The toxicity mechanism is not determined yet [7].
Herein, we report a case with crystalline retinopathy that is the first report of crystalline retinopathy due to Oxaliplatin toxicity.
Case Report
A 72-year-old lady, known case of colorectal cancer under treatment with Oxaliplatin on a Capox schedule for two consecutive years was referred to our hospital because of decreased visual acuity and ocular surface symptoms. Visual impairment was mainly in right eye. Her uncorrected visual acuity was 4/10 in OD and 5/10 in OS (snellen chart) and following refraction her best corrected visual acuity increased to 6/10 in OD (+0.75-0.5 ×75˚) and 10/10 in OS (+1.25-1.00 ×95˚). In slit lamp examination, bilateral small pterygium and mild to moderate cataract (nuclear sclerosis) detected. Scattered yellowish iridescent dots with diffuse pigmentary changes through retina were observed in her both eyes in funduscopy (Figure 1).
Other paraclinic tests requested afterward due to suspicion of two differential diagnoses (toxic retinopathy and cancer associated retinopathy).
Blue Autofluorescence (BAF) and Infrared (IR). Multiple extensive hypofluorescence dots with different sizes in posterior pole were revealed in BAF of both eyes that were compatible with iridescent dots. These alternations were less visible in IR as compared with BAF and fundus photograph (Figure 1).
Optical coherence tomography. Outer retinal atrophy, severe retinal thinning, mottling and disruption of ellipsoid zone and irregular RPE damage were demonstrated on OCT imaging of both eyes in first exam (Figure 2). These changes were more severe in right eye. After discontinues of oxaliplatin, OCT was performed again and all changes were stable in OCT without any recovery (Figure 2).
Electoretinography and Electrooculography. Photopic and scotopic ERG tests were requested for the patient. Severe reduced response of scotopic ERG and moderate reduced response in photopic ERG were detected in first visit of patient. Three months later after discontenting drug, ERG was performed again and improvement in response was detected in second time (Figure 3). EOG was also performed in second visit for patient and the significant reduction in ARDEN ratio observed in both eyes (123% in OD and 145% in OS).
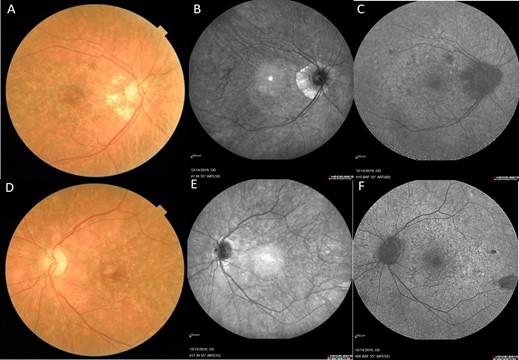
Figure 1: Fundus photograph (A and D), infrared (B and E) and fundus autoflourescent (C and F) of our patient. Scattered yellowish iridescent dots in posterior pole of both eyes, diffuse pigmentary changes, pale disk and peripapillary atrophy especially in right eye are observed in fundus photograph. Mild changes (few hyper-reflective dots) are seen in infrared. Extensive hypo-fluorescent spots with different sizes in association with some hyper-fluorescent dots are seen in fundus autofluorescent.

Figure 2: Optical coherence tomography (OCT) of our patient in first exam (yellow framework) and second exam (red framework) after 3 months. Retinal thinning, outer retinal atrophy, mottling and irregular disruption of ellipsoid zone and irregularity of retinal pigment epithelium were seen in first OCT and were stable without any recovery in second OCT.
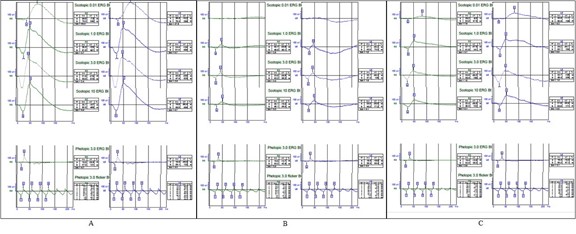
Figure 3: Electroretinography (ERG) of normal person (A) and our patient in first (B) and second (C) visits. Both scotopic and photopic response of our patients reduced significantly (B) and after 3 months of discontinuation oxaliplatine, significant improvement was seen in ERG response (C).
Discussion
Oxaliplatin is a platinum-based drug and is prescribed in combination with fluorouracil and leucovorin in a famous combination known as Folfox which is one of standard treatments for advanced colorectal cancer. [8,9] Ocular side effects of Oxaliplatin are rare and clinicians usually notice to other possible side effects of this drug like neurotoxicity or hematologic adverse effects. Oxaliplatin has been reported to induce different ocular impairments, most of them are reversible and in some cases, it has been permanent [1,6].
In a case report by Mesquidaa et al. for the first time a patient with permanent damage to RPE following Oxaliplatin administration is reported. There has been no alteration in the retina in funduscopy but in visual field examination bilateral concentric scotoma was reported with diminished EOG response in both eyes and OCT as well as ERG had normal results [10]. Our patient also showed permanent damage, and it thus differs from previously described Oxaliplatin induced ocular toxicities, which are usually transient and reversible. It may be related to delay in diagnosis. The changes in OCT were stable without any recovery in our patient, although her ERG response showed some improvement. It might be related to some degrees of recovery that is revealed as functional improvement without any significant structural changes. On the other hands, the recovery in structure maybe needs more time. Early intervention and cessation of the drug would improve the condition and cause reversible changes.
Our case is the second report of RPE damage following Oxaliplatin administration but with new findings (crystalline retinopathy). It is the first case showing iridescent white dots spread through funds in both eyes and also outer retina and RPE atrophy in OCT of both eyes.
In Oxaliplatin pharmacolo-chemistry there is oxalic acid components and chlorine ligands are replaced by the oxalato bidentate derived from oxalic acid for water solubility improvement and it may explain the yellow dot pattern and in fact possible crystalline retinopathy which was visible in our patient.[9] Crystalline retinopathy was reported in several conditions like chronic retinal detachment, primary hyperoxaluria, Bietti crystallin dystrophy, talc emboli, and cystinosi or following specific chemical medications like canthaxanthin, methoxyflurane, tamoxifen, nitrofurantoin and etc [11]. Due to component of Oxalipaltin, occurrence of crystalline retinopathy can be possible in administration of this drug, although it has not been reported previously. This is the first report of crystalline retinopathy due to Oxaliplatin usage. In crystallin retinopathy associated with methoxyflurane toxicity dose related deposition of oxalate crystals occurs in retina. In examination yellow crystals are visible often in an arterial distribution throughout the retina, at the level of the RPE [12]. Our patient had scattered yellowish iridescent dots in posterior pole of both eyes and also anterior to arcades which were more visible around arteries (Figure 1).
In primary hyperoxaluria an autosomal recessive disease with overproduction of oxalate in body, fundus autofluorescence scattered hyper auto fluorescent dots corresponding to crystals will be observed and in SD-OCT images hyper reflective spots in regions of RPE elevation will be found in addition to atrophic changes of macula.[12] In our case SD-OCT images showed outer retina and RPE atrophy mainly in macula with scattered hyper-autoflurescent spots not only through outer retina and RPE but also choroid as its mentioned in some oxalate retinopathy conditions like primary hyperoxaluria [12].
It is difficult to establish an association between a specific drug and visual disturbances because of the low incidence of these side effects and also existence of multiple simultaneous factors which can cause ocular sign and symptoms.
One of the most important differential diagnosis of our case was cancer associated retinopathy or CAR. Like toxic retinopathy associated with Oxaliplatin there is no single diagnostic criterion for CAR. The clinical symptoms in CAR have a usually acute or sub-acute presentation. It is usually diagnosed according to clinical suspicion of normal funds at the early periods of the disease in spite of severe ERG response attenuations, positive anti-retinal antibodies in lab date, and atrophic changes in RPE [13].
Symptoms in our case were not so acute and progressive and also it regressed following oxaliplatin cessation, we didn’t test antirational antibody for the patient because of economic burden of this test for the patient and that more than 40% of the patients with CAR will have negative autoantibodies against retinal antigens. Also, positive tests for these autoantibodies are reported in healthy individuals [14]. Significant improved ERG response after 3 months after discontinuation of Oxaliplatin usage strengthened the diagnosis of drug toxicity other than CAR. On the other hand, improvement of ERG response without any intervention was not compatible with the diagnosis of CAR. Decreased ERG response could be associated with minimal changes in fundus on the contrary our case. Reduction in Arden ratio usually is mild in CAR in contrast our patient.[14] Overall, these findings coincided with the diagnosis of drug toxicity other than CAR.
Conclusion
Our case is the first report of crystalline retinopathy following Oxaliplatin administration with concomitant outer retina and RPE damage.
In spite of its low frequency, toxic adverse effect of this drug can impact permanent damage of ocular structure and cause visual impairment if being neglected and clinicians should pay more attention to this rare but possible side effect of this medication group.
Disclosure Statement: The authors do not have any financial conflict of interest.
Patient Consent: The patient has consented to the submission of the case report for submission to the journal.
Declaration:
This manuscript has not published or submitted for publication elsewhere.
Authors declare any financial support or relationships that may pose conflict of interest.
References
- Wiseman LR, Adkins JC, Plosker GL, Goa KL. Oxaliplatin: a review of its use in the management of metastatic colorectal cancer. Drugs Ageing, 1999; 14(6): 459-475.
- Cassidy J, Misset JL. Oxaliplatin-related side effects: characteristics and management. Semin Oncol, 2002; 29: 11–20.
- Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs, 2003; 63(15): 1549-1563.
- O’Dea D, Handy C, Wexler A. Ocular changes with oxaliplatin. Clin J Oncol Nurs, 2006; 10: 227–229.
- Zhang RX, Lu ZH, Wan DS, Wu XJ, Ding PR, Kong LH, et al. Neuroprotective e ect of neurotropin on chronic oxaliplatin-induced neurotoxicity in stage II and stage III colorectal cancer patients: results from a prospective, randomised, single-centre, pilot clinical trial. Int J Colorectal Dis, 2012; 27(12): 1645-1650.
- Simpson D, Dunn C, Curran M, Goa KL. Oxaliplatin, a review of its use in combination therapy for advanced metastatic colorectal cancer. Drugs, 2003; 63: 2127–2156.
- Imperia PS, Lazarus HM, Lass JH. Ocular complications of systemic cancer chemotherapy. Surv Ophthalmol, 1989; 34: 209–230.
- De Gramont A, Figer M, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol, 2000; 18: 2938-2947.
- Graham J, Mushin M, Kirkpatrick P. Oxaliplatin. Nat Rev Drug Discov, 2004; 3(1): 11-12.
- Mesquidaa M, Dalmaua BS, Pereza SO, Molina-Fernandeza JJ, Pelegrína L, Roca MF, et al. Oxaliplatin-Related Ocular Toxicity. Case Rep Oncol, 2010; 3: 423–427.
- Ahmed I, McDonald HR, Schatz H, Johnson RN, et al. Crystalline Retinopathy Associated with Chronic Retinal Detachment. Arch Ophthalmol, 1998; 116: 1449-1453.
- Kovach JL, Isildak H, Sarraf D, Crystalline Retinopathy. Unifying Pathogenic Pathways of Disease, Survey of Ophthalmology, 2018. doi: 10.1016/j.survophthal.2018.08.001.
- Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol, 2003; 48: 12-38.
- Naramala S, Ahmad J, Adapa, et al. Case Series of Cancer-associated Retinopathy (CAR). Cureus 11(6): e4872.

