Five-Year Survival in Glioblastoma Patients Treated by Radio-Chemotherapy Alone or in Association with a Neuroendocrine Approach Consisting of the Pineal Hormones Melatonin, Methoxytryptamine, and Pinealine Plus Cannabidiol
Paolo Lissoni*, Franco Rovelli, Giusy Messina, Marco Tomasi, Luca Viganò, Dino Ceppodomo and Giuseppe Di Fede
Institute of Biological Medicine, International Institute of Pnei, Italy
Received Date: 04/08/2023; Published Date: 15/12/2023
*Corresponding author: Paolo Lissoni, Institute of Biological Medicine, International Institute of Pnei, Milan, Italy
Abstract
At present, it is known that the prognosis of the neoplastic diseases does not depend only on tumor genetic characteristics, but also on patient immunobiological status, which is synthetically the result of the interactions occurring between endogenous pro-tumor and antitumor molecules. In the case of glioblastoma (GBM), it may inhibit by several pineal hormones, as well as by the cannabinoid agents. On these bases, a study was planned to evaluate the impact of a concomitant regimen with pineal hormones and Cannabidiol (CBD) on the clinical response and survival of GBM patients underwent a partial tumor resection. The study included 80GBM patients, who were randomized to receive the only standard treatment with radiotherapy plus temozolomide, or in association with a neuroendocrine combination containing the pineal hormones melatonin (MLT), 5-Methoxytryptamine (5-MTT), and Pinealine (PNL) in association with the cannabinoid agent cannabidiol (CBD). All agents were orally administered. MLT was given at high-doses (100 mg/day in the night), 5-MTT at 10 mg/day during the light phase, PNL at 1 mg/day in the evening, and CBD at 10 mg twice/day. Both objective tumor regressions and 5-year survival were significantly greater in patients concomitantly treated by the neuroendocrine schedule. Moreover, irrespectively of the type of therapy, both lymphocyte mean count and Lymphocyte-to-Monocyte Ratio (LMR) mean values observed on treatment were significantly higher in patients who achieved a disease control than in those, who progressed on therapy. In conclusion, this study shows that the efficacy of the treatment of GBM patients may be improved by the concomitant administration of both endogenous and exogenous natural anticancer molecules, such as pineal hormones and cannabinoids. Moreover, the results of this studyon the basis of changes in LMR values, would suggest that the immune response plays a role in the efficacy of treatment. Further studies will be required to establish which may be the best cannabinoid agent in the treatment of GBM.
Keywords: Brain radiotherapy; Cannabidiol; Glioblastoma; Lymphocyte-to-monocyte ratio (LMR); Pineal hormones; Temozolomide
Introduction
Even though several clinical studies have been performed with all potentially effective anticancer therapies, including chemotherapy, targeted therapy against tumor growth factor receptors, anti-angiogenic treatments, and different kinds of immunotherapy, brain glioblastoma (GBM) remains the most untreatable human neoplasm. Then, the only partially active agent is the chemotherapeutic agent Temozolomide (TMZ). The less curability of GBM would depend at least in part on the fact that no therapeutic protocol has been elaborated up to now by considering the endogenous molecules involved in the stimulation and inhibition of GBM tumor cells. On the contrary, it is known since many years that brain tumor growth is stimulated by the mu-opioid agonists [1], including beta-endorphin and morphine, whereas it is inhibited by the pineal indole melatonin (MLT) [2] and cannabinoid endogenous and exogenous agents [3]. MLT and cannabinoid agents may opposite cancer growth by a direct antiproliferative cytotoxic action, or through a modulation of the immune system in an antitumor way, then by influencing lymphocyte and macrophage tumor infiltration, which has been proven to influence the prognosis of tumours [4,5]. In more detail, tumor prognosis is better in the presence of Th1 and cytotoxic T lymphocytes into tumor mass, whereas it is worse when tumor infiltration is preferentially constituted of macrophages, regulatory T (T reg) [4,5] and Th17 [6,7] lymphocytes. Lymphocyte count has appeared to correlate with the efficacy of the antitumor immunity, since lymphopenia has been proven to predict a lower survival and resistance to the anticancer therapies [8]. On the contrary, monocyte count positively correlates with macrophage tumor infiltration [4,5], and its increase reflects a poor prognosis. Then, the evidence of an abnormally low values of Lymphocyte-to-Monocyte Ratio (LMR) has been proven to predict a poor prognosis in almost all human neoplasms [9]. While cancer chemotherapy may induce both decline or increase in lymphocyte count with respect to the neutrophil number [10], Radiotherapy (RT), particularly pelvis and mediastinum irradiation, constantly determines lymphocytopenia, with the only exception of brain irradiation, which in contrast could enhance lymphocyte count [11]. Moreover, RT-induced lymphocytopenia has appeared to negatively influence the prognosis of most human neoplasms. Then, because of the stimulatory effect of MLT on Th1-related secretion of IL-2 [12], the main growth factor for T lymphocytes [13], the concomitant treatment with high-dose MLT could counteract cancer-related lymphocytopenia, while cannabinoids have no relevant effect on lymphocytes, since their anti-inflammatory activity would be mainly due to inhibition of macrophage system [14]. Moreover, MLT is not the only anticancer hormone produced by the pineal gland, since at least two other antitumor hormones have been identified, the indole 5-methoxytryptamine (5-MTT) [15,16], and the beta-carboline 6-methoxy-1,2,3,4 tetrahydro-beta carboline, also termed as pinealine (PNL), which is also provided by expansional mind property [17]. On these bases, a randomized study was performed to evaluate the impact of a concomitant neuroendocrine regimen with MLT, 5-MTT, PNL, and CBD on the survival time and on changes in LMR with respect to radio-chemotherapy alone in patients suffering from GBM.
Patients and Methods
The study included 80 consecutive patients (M/F: 56/24; median age 54 years, range 28-78 years) affected by GBM, who underwent macroscopically partial tumor resection. After the approval by the Ethical Committee, the clinical protocol was explained to each patient, and written consent was obtained. Eligibility criteria were, as follows: histologically proven GBM, measurable lesions, palliative surgery with macroscopically evident persistence of disease, and no double tumour. After partial surgery, according to the standard treatment, patients underwent radio-chemotherapy with brain irradiation plus TMZ. RT consisted of 60 Gy at 2 Gy/day fractions, in association with TMZ at 25 mg/m2/day orally. Patients were randomized to receive RT plus TMZ alone, or in association with a neuroendocrine regimen consisting of MLT plus CBD, 5-MTT, and PNL. All agents were given orally. MLT was administered at 100 mg/day in the night, according to its light/dark rhythm. 5-MTT was given at 10 mg/day during the period of maximal light. PNL was administered at 1 mg at 8 PM. Finally, CBD was given at 10 mg twice/day (8 AM and 8 PM). The treatment was continued without interruption. NMR was made before and after at least 40 days from the end of RT, then it was repeated at 3-month intervals for the first two years, and at 6-month intervals for the successive period. LMR values were detected before, after 15 days and at the end of brain irradiation. Normal values observed in our laboratory (95% confidence limits) were, as follows: lymphocytes: above 1,000/mm3; monocytes: below 450/mm3; LMR: more than 2.1. The clinical response was evaluated according to WHO criteria. Data were reported as mean +/- SE, and statistically evaluated by the student’s t test, the analysis of variance, and the chi-square test, as appropriate. Finally, the survival curves were elaborated according to the Kaplan-Meyer method, and statistically assessed by the log-rank test.
Results
The clinical response obtained in the two groups of patients is reported in Table 1. As shown, both percentages of objective tumor regressions, including Complete Response (CR) and Partial Response (PR), and Disease Control (DC) were significantly higher in patients concomitantly treated with the neuroendocrine regimen than in those treated by the only radiotherapy and chemotherapy (P<0.05 and P<0.025). Moreover, as illustrated in Figure 1, the percentage of five-year survival achieved in patients concomitantly treated with the neuroendocrine regimen was significantly greater than that found in those treated with the only standard therapy (P<0.05). Table 2 shows the immune changes occurred in patients in relation to their clinical response. Irrespectively of the type of therapy, lymphocyte mean number significantly increased on therapy (P<0.05), whereas it remained substantially unchanged in patients with SD and significantly decreased in those who had a PD (P<0.05). On the other side, monocyte count remained substantially unchanged. Then, LMR mean values observed in patients with objective tumor regression at the end of RT were significantly higher than those found prior to therapy (P<0.05) and after therapy in patients with PD (P<0.025). LMR mean values increased on therapy also in patients with SD, without, however, significant differences. On the contrary, LMR mean values decreased on therapy in patients with PD, even though to the difference was not statistically significant.
Discussion
The results of this study show that the efficacy of radio-chemotherapy for GBM may be enhanced by a concomitant administration of a neuroendocrine regimen consisting of natural molecules, such as pineal antitumor hormones and cannabinoid agents, provided by inhibitory action on brain tumor growth, and immunostimulatory effects on the anticancer immunity, as demonstrated by an increase in LMR values in patients, who achieved a disease control or tumor regression on therapy. Then, the prognosis of GBM may be predicted not only on the basis of the genetic characteristics of tumours, but also by the antitumor immune response of patients. Obviously, further studies by detecting the different lymphocyte subsets will be required to better define the immune status of patients, mainly Th1, T reg ad Th17 cells, but from a clinical point of view the simple determination of LMR would be sufficient to evaluate the antitumor immune status of patients, as previously shown by other authors [9]. If successive studies will demonstrate that LMR variations on therapy are not a simple epiphenomenon, but a clinical variable provided by pathogenetic and prognostic significance, endocrine and immune approaches capable of opposing lymphocyte decrease and monocyte increase could allow a greater efficacy of radio-chemotherapy. Then, the results of this preliminary study would justify further randomized clinical studies in GBM with radio-chemotherapy alone versus radio-chemotherapy in association with a neuroimmune regimen, consisting of the pineal hormone MLT and cannabinoid agents. Moreover, the results of this study show that the enhanced tumor regression rate induced by a concomitant administration of the neuroendocrine antitumor combination is associated with a statistically significant longer survival with respect to that achieved by the only standard radio-chemotherapeutic treatment. However, it is important to remarked that the neuroimmune regimen employed in this study has been limited to the only administration of anticancer pineal indoles and cannabinoid agents. Then, the efficacy of the neuroimmune regimen could be furtherly enhanced by associating another fundamental endogenous molecule, the angiotensin 1-7 (Ang 1-7), the enzymatic product of ACE2, which has recently appeared to exert an important anticancer action by inhibiting both cancer cell proliferation and angiogenesis [18,19], as well as IL-2 [13], the main cytokine responsible for the generation of an effective anticancer immunity. Finally, future studies will be needed to establish which is the most effective anticancer cannabinoid, since preliminary results would suggest a greater efficacy of Cannabigerol (CBG) with respect to CBD, at least in the treatment of GBM [20]. Then, CBG would also be included within the neuroendocrine approach to the treatment of GBM. In any case, the results of this study justify further clinical investigation to identify the optimal neuroendocrine and immune regimen to be associated with the standard oncological therapy of GBM, as well as of other brain neoplasms.
Table 1: Clinical response (WHO criteria) obtained in GBM patients treated by radiotherapy and chemotherapy alone (group A), or in association with MLT, 5-MTT, PNL, and CBD (group B).
Table 2: Mean values (+/-SE) of lymphocyte, monocyte count (n/mm3) and lymphocyte-to-monocyte ratio (LMR) in 80 GBM patients undergoing radio-chemotherapy in relation to their clinical response (CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease).

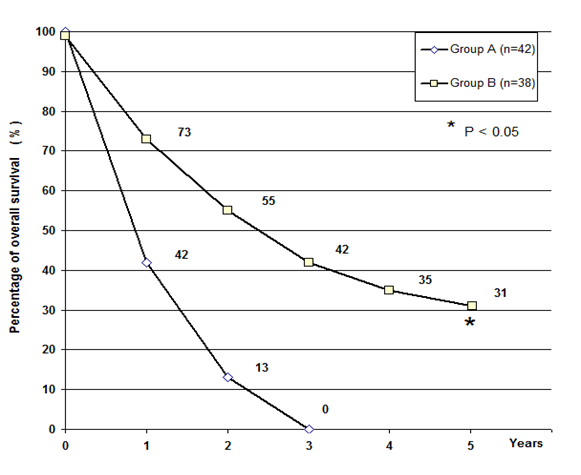
Figure 1: Five-years survival in glioblastoma patients treated by the only radio-chemotherapy (Group A) or in association with pineal hormones plus cannabidiol (Group B).
References
- Westphal M, Li CH. Beta-endorphin characterization of binding sites for the human hormone in human glioblastoma SF 126 cells. Proc Natl Acad Sci USA, 1984; 81: 2921-2923.
- Reiter RJ. Mechanisms of cancer inhibition by melatonin. J Pineal Res, 2004; 37: 213-214.
- Blazquez C, Salazar M, Carracedo A, Lorente M, Egia A, Gonzales-Feria L, et al. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res, 2008; 68: 1945-1952.
- Mantovani BA, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature, 2008; 454: 436-444.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell, 2010; 295: 883-889.
- Wang S, Li Z, Hu G. Prognostic role of intratumoral IL-17A expression by immunohistochemistry in solid tumors: a meta-analysis. Oncotarget, 2017; 8: 66382-66391.
- Chang SH, T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch Pharmacol Res, 2019; 42: 549-559.
- Riesco A. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer, 1970; 25: 135-140.
- Gu L, Li H, Chen L, Ma x, Li X, Gao Y, et al. Prognostic role of lymphocyte-to-monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget, 2016; 3: 7876-7881.
- Ehrke MJ, Mihich E, Berd D, Mastrangelo MJ. Effects of anticancer drugs on the immune system in humans. Semin Oncol, 1982; 16: 230-239.
- Louagie H, van Eijkeren M, Philippe J, Thierens H, de Ridder L. Changes in peripheral blood lymphocyte subsets in patients undergoing radiotherapy. Int J RadiatBiol, 1999; 75: 767-771.
- Maestroni GJM. Theimmunoneuroendocrine role of melatonin. J Pineal Res, 1993; 14: 1-10.
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. J Exp Med, 1982; 155: 1823-1841.
- Grotenhermen F. Pharmacology of cannabinoids. Neuroendocrinol Lett, 2004; 25: 14-23.
- Nagarkatti P, Pandey R, Rieder SA, Hedge VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem, 2009; 1: 1333-1349.
- Sze SF, Ng TB, Liu WK. Antiproliferative effect of pineal indoles on cultured tumor cell lines. J Pineal Res, 1993; 14: 27-33.
- Di Fede G, Messina G, Monzon A, Meli O, Gavazzeni C, Rovelli F, et al. Clinical effects of the pineal antitumor and psychedelic beta-carboline pinealine in the palliative therapy of untreatable metastatic cancer patients. Integr Cancer Biol Res, 2017; 1: 2-5.
- Capettini LS, Montecucco MF, Mach F, Stergiopulos R, Robson SA, da Silva RF. Role of renin-angiotensin system in inflammation, immunity, and aging. Curr Pharm, 2012; 18: 963-970.
- Gallagher PE, Arter AL, Deng G, Tallant EA. Angiotensin-(1-7): a peptide hormone with anticancer activity. Curr Med Chem, 2014; 21: 2417-2423.
- Lah TT, Novak M, Almidon MAP, Marinelli O, Baskovic BZ, Majc B, et al. Cannabigerol is a potential therapeutic agent in a novel combined therapy for glioblastoma. Cells, 2021; 10: 340-346.

