Primary Esophageal Lymphoma: Clinical Experience in Diagnosis and Treatment
Vivek.G Nath*, Govindaraj Raman Senthilkumaran, V C Kalyanasundara Bharati, Joel Kumar Earjala and U Aravindan
Senior Resident, Department of Surgical Gastroenterology, Thanjavur Medical College, Tamil Nadu, India
Received Date: 09/07/2023; Published Date: 04/12/2023
*Corresponding author: Dr. Vivek G Nath, Senior resident, Department of Surgical Gastroenterology, Thanjavur medical college, Tamil Nadu, India
Abstract
Gastrointestinal system is the most affected extra-nodal site for lymphoma, with primary oesophageal lymphoma as a rare diagnosis. It is highly nonspecific clinically and indistinguishable from other benign and malignant conditions. We present a middle-aged male with dysphagia and weight loss with a submucosal lesion in the lower oesophagus in endoscopy. Initial evaluation and biopsy pointed towards oesophageal lymphoma/NET with a suspected liver secondary in PET CT. Patient underwent video assisted thoracoscopic esophagectomy with two field lymphadenectomy and left lateral sectionectomy. Post operative histopathological examination and IHC analysis revealed primary Diffuse large B-cell lymphoma of oesophagus (R0 resection) with fatty changes in liver. Patient was kept on surveillance after a postoperative PET CT, which showed no residual disease.
Keywords: Esophageal lymphoma; DLBCL; Dysphagia; Minimal invasive esophagectomy; Malignancy
Introduction
Gastrointestinal (GI) lymphoma comprises about 5–20% of extra-nodal lymphomas [1]. The most common location being stomach, followed by small intestine (ileum (60–65%), jejunum (20%−25%), and duodenum (6%–8%) and then colorectum (6–12%)) [2]. Generally described risk factors implicated in the pathogenesis of GI lymphoma are some infections due to Helicobacter pylori, human immunodeficiency virus infection, Campylobacter jejuni, Epstein-Barr virus, hepatitis B virus, human T-cell lymphotropic virus-1 [3]. Some inflammatory conditions such as celiac disease, inflammatory bowel disease, atrophic gastritis, and parasitic infection can also be attributed to the development of GI lymphoma [4]. Oesophageal primary lymphoma is extremely rare and hereby we discuss a case report with review of literature.
Case Presentation
59-year-old man presented to our OPD with dysphagia, regurgitation, and unintentional weight loss of 10 kg for 6 months. Dysphagia was progressive in nature and grade 4 on presentation, associated with regurgitation of food mixed with saliva. He does not have any history of pain abdomen, odynophagia, alteration in bowel habits or fever. No significant medical or surgical history in the past. He is an occasional alcoholic, smoker and with mixed food habits. On clinical examination he was afebrile, pale. He was obese (BMI-30.6) and had moderate dehydration. There was no generalised lymphadenopathy or oedema. Chest and abdomen examinations were normal and routine blood investigations were also within normal limits. The patient was admitted and optimised with intravenous fluids, anti-emetics, and nutrition.
Upper GI endoscopy was done which revealed a submucosal long segment bulging growth in the mid & lower oesophagus with difficulty in negotiating scope to stomach.
He had done Multiple biopsies with inconclusive results and eventually a diagnosis of NET oesophagus/ lymphoma was made by biopsy. The sections showed hyperplastic squamous epithelium with underlying neoplasm composed of solid sheets of monotonous population of cells with round nuclei and scant cytoplasm infiltrating the stroma.
The finding was supported by focal synaptophysin positive in IHC. The Serum chromogranin level was also found to be elevated.
A contrast enhanced CT of thorax with abdomen done showed proximal dilated oesophagus with air fluid level. There was also a large mildly enhancing polypoidal mass along the right lateral and anterior walls of the mid and distal thoracic oesophagus extending to the GE junction, causing luminal narrowing, measuring 10.2 cm in craniocaudal dimension, 5.6 cm in transverse dimension and 5 cm in anteroposterior dimension. The periesophageal fat planes were preserved with no infiltration of the adjacent organs. A few enlarged periesophageal, subcarinal and left gastric lymph nodes were seen, with largest in the left gastric group measuring 10 x 7 mm (Figure 1a).
PET CT revealed 10.2 x 5.6 x 5 cm hypermetabolic polypoidal mass mid and distal thoracic oesophagus extending to the GE junction, suggestive of oesophageal malignancy (SUV max-4.22). No adjacent mediastinal organ infiltration was seen. Few periesophageal, subcarinal and left gastric lymph nodes with mild metabolism, likely metastatic lymphadenopathy was noted (Figure 1a).
There was a hypermetabolic focal hypodense lesion in the left lobe of the liver (SUV max-6.23), measuring 1.4 x 1.2 cm suspicious of hepatic metastasis (Figure 1b).
A preoperative diagnosis of NET oesophagus with liver oligo-metastasis was made provisionally. After optimisation and preoperative cardiopulmonary fitness evaluation, proceeded with minimal invasive esophagectomy and left lateral sectionectomy.
Intra operative findings showed dilated esophagus with distal growth extending to OGJ. There was no ascites, peritoneal deposits. Liver was found to be steatotic. Esophagectomy and gastric conduit placement was done with cervical esophago-gastric anastomosis along with left lateral sectionectomy and feeding jejunostomy (Figure 2). Post operative recovery was uneventful, and the patient was discharged full form on pod-12 with oral soft diet tolerating.
Post operative specimen examination shows a circumscribed grey, white homogenous solid mass measuring 11x4.5x3.5 cm in submucosa of distal esophagus; situated 6.5 cm from proximal end and 4 cm from distal end (Figure 3). Grossly resected margins are free.
Histopathological study showed a submucosal neoplasm composed of monotonous population of small to medium sized cells arranged in nests with intervening fibrous septa infiltrating the muscularis propria. Individual cells had scant cytoplasm, irregular nuclei, dense chromatin, and inconspicuous nucleoli (Figure 4A & 4B).
Immuno-histochemistry study showed synaptophysin negative, CD-3 negative and CD 20 positive (Figure 4c&4d). Section studied from liver showed fatty change with periportal and bridging fibrosis, inflammation with no evidence of dysplasia.
This was suggestive of primary Esophageal Non-Hodgkin’s lymphoma-diffuse b cell type. A follow up PET scan was performed, which revealed no residual lesion and patient is kept in close follow up.
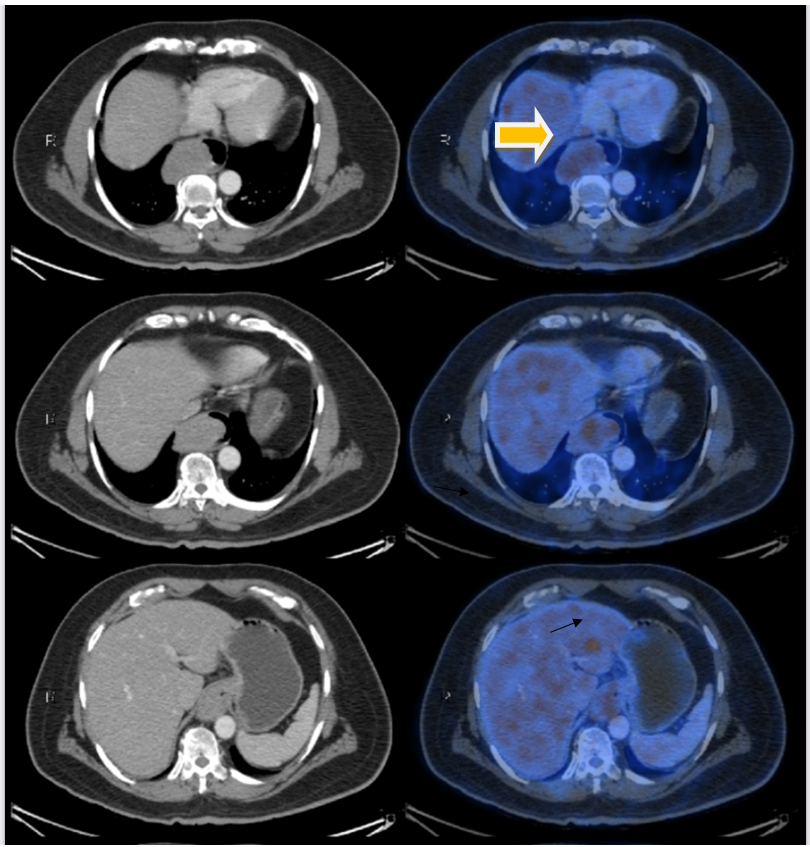
Figure 1a: PET CT imaging showing lower oesophageal growth (yellow arrow) and suspected metastatic lesion in liver (black arrow).
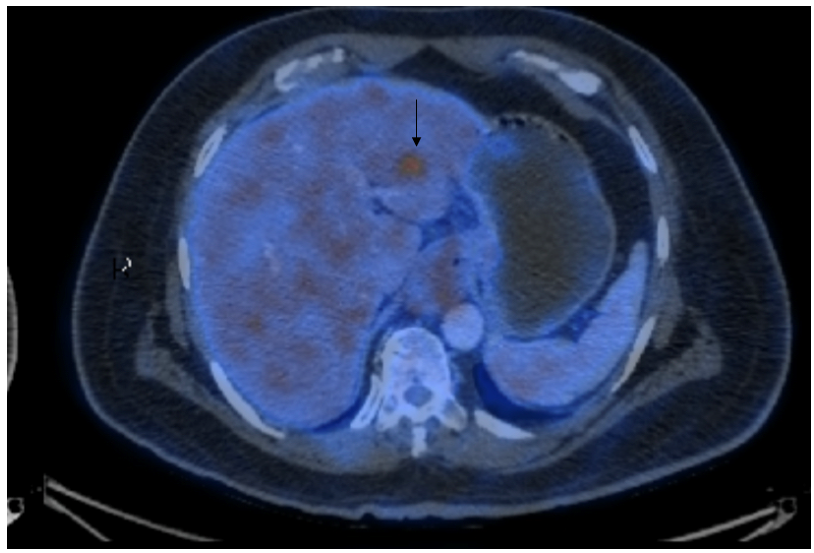
Figure 1b: black arrow showing suspected liver metastasis.

Figure 2: gross resected specimen of esophageal lymphoma with left lateral sectionectomy.

Figure 3

Figure 4A: 1OX view of neoplasm.
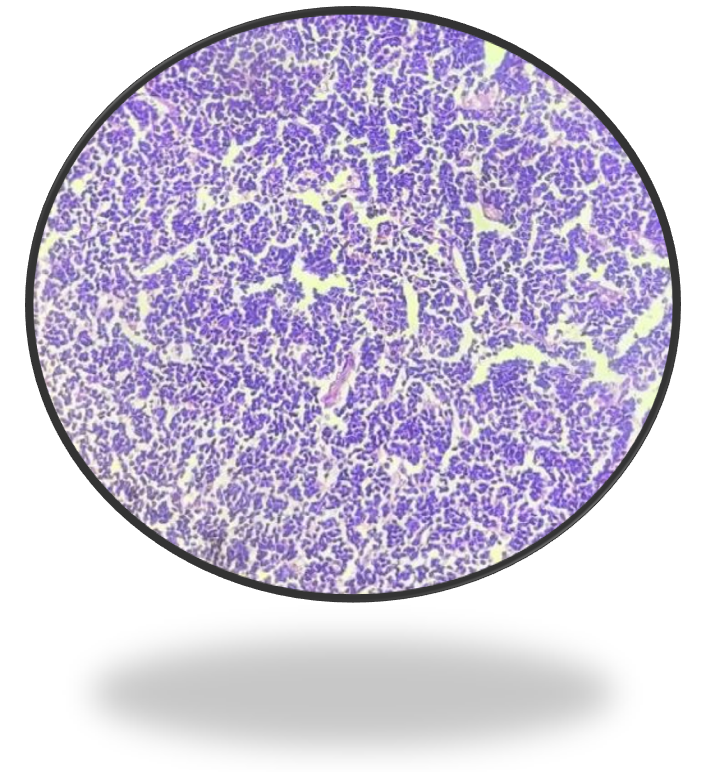
Figure 4B: 40X View of neoplasm.
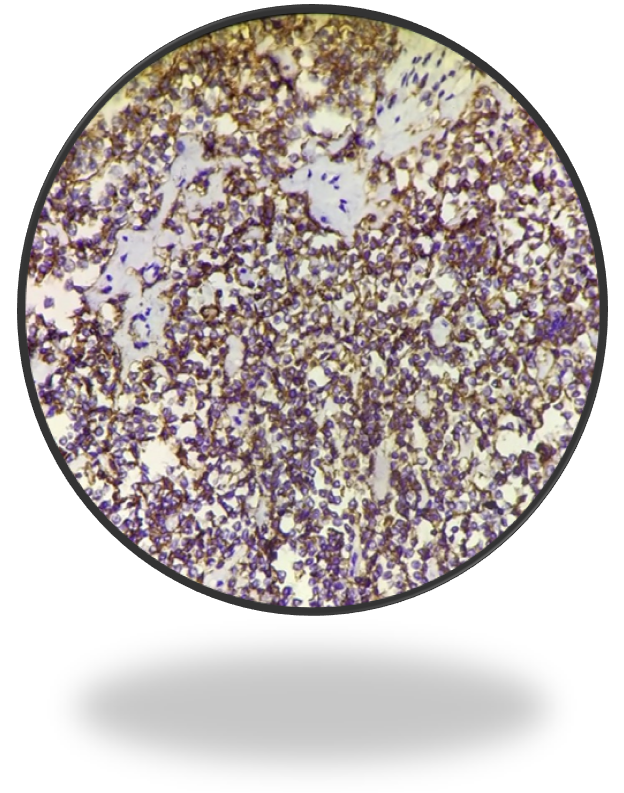
Figure 4c: IHC- CD 20 positive.

Figure 4d: IHC CD 3 negative.
Discussion
Diffuse large B cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma, comprising about 30–40% of all cases world-wide. It is Characterised with a rapidly growing tumour mass in single or multiple, nodal or extra-nodal sites, with not otherwise specified as the most common subtype, which represents 80–85% of all cases [5]. 5th edition of the World Health Organization Classification of Haematolymphoid Tumours in 2022 focused on lymphoid neoplasms had modified the name from “diffuse large B-cell lymphoma “to “large B-cell lymphoma“, acknowledging the fact that a diffuse growth pattern is either not present or cannot be assessed in some entities (e.g., fibrin-associated large B-cell lymphoma or fluid-overload associated large B-cell lymphoma) [6]. DLBCL is a disease of elderly population with a median age in the 7th decade and with rare occurrence among young adults and children. A slight male predominance is also seen in the condition. DLBCL present in localized manner in approximately 20% of patients. Disseminated extra-nodal disease is uncommon, and one third of patients presents with systemic symptoms. Even though DLBCL are aggressive, they are potentially curable malignancies [7]. Primary Gastrointestinal (GI) lymphoma is a rare clinically nonspecific entity which are difficult to distinguish from other benign and malignant GI lesions. Primary GI Lymphoma can involve any part of the GI tract, frequent sites in order of its occurrence are the stomach followed by small intestine and ileocecal region [3]. Oesophageal involvement has been reported in less than 1% of cases and mostly occurs as an extension of mediastinal or gastric lymphoma. Primary oesophageal B Cell lymphoma is extremely rare with very few case reports in the literature [8]. Even though our case satisfies the Dawson’s criteria, strict application of Dawson’s criteria is controversial in different clinical settings.
Clinical diagnosis of primary oesophageal lymphoma is extremely difficult with variable clinical symptoms, imaging, and upper GI endoscopy findings. It is hard to prove the disease with a biopsy due to its submucosal location of the tumor. Clinical manifestation can vary from a range variable presentation like protruding a mass, submucosal nodule, ulcer, thickening etc [9]. Introduction of EUS guided biopsy has made great progress in the diagnostic accuracy of oesophageal lymphomas [10] Contrast enhanced CT of thorax and abdomen may help in differentiating primary oesophageal lymphoma from lymph node involvements in the cervical or mediastinal regions, in staging of the disease, ruling out complications and in evaluating treatment therapy [11].
The imaging modality of choice for staging and follow-up in Hodgkin disease and most non-Hodgkin lymphomas is 2-[fluorine-18] Fluoro-2-Deoxy-d-Glucose (FDG) Positron Emission Tomography (PET) scan. Considering GI lymphoma, FDG uptake is different according to the different location with oesophageal lymphoma manifests as circumferential thickening of the oesophageal wall with increased FDG uptake [12].
To confirm the diagnosis of DLBCL, one must confirm the B-cell lineage of the lymphoma cells, with expression of CD20. In untreated lymphomas, CD20 is expressed in the most mature B-cell neoplasms, with some notable exceptions. Monoclonal antibody therapy (rituximab) targets CD-20 which is present in most B-cell lymphomas. Rituximab-treated B-cell lymphomas may not express CD20, which requires additional immune-stains, like PAX5, CD79a, CD19, or CD22, to identify B cells [13].
Surgical intervention may be warranted in case of an oesophageal lymphoma in the background of obstruction, bleeding, perforation, or diagnostic dilemma. Minor stenosis can be managed with endoscopic dilation [14]. Chemotherapy and radiotherapy are the mainstay of treatment with very favourable outcome depending on the type of the lymphoma especially the CHOP regimen. Addition of rituximab with CHOP regimen have shown a higher treatment response rate and better outcome for DLBCL patients [15]. External beam radiation at the dose of 40 Gy can also be used in the management of oesophageal lymphoma [16]. Post operative chemotherapy, radiotherapy or both can increase the survival and reduce recurrence. Early stage tumors, without lymphadenopathy, low and intermediate grade, size <10 cm with complete excision can be observed without adjuvant therapy [17]. Extra nodal DLBCL tends to be localised to the anatomical site, hence prognostically more favorable than nodal equivalents.
Conclusion
Understanding of primary GI lymphoma, especially esophageal lymphoma, had major advancements in the past two decades. EUS guided stacked biopsy and cutting-edge imaging helped in precise histopathological diagnosis and proper staging of disease. Extra nodal DLBCL confined to anatomical sites have better prognosis when managed with a chemotherapy, radiotherapy, or both. Surgical management is indicated in the presence of complications and is curative when R0 resection is achieved.
Authorship criteria
- Vivek G Nath-concept, design, draft, guarantor, correspondence.
- Govindaraj Raman Senthilkumaran- concept, design, draft
- V C Kalyanasundara Bharati- concept, design, draft
- Joel Kumar Earjala- concept, design, draft
- Prof U Aravindan- concept, design, draft
Conflicts of Interest/ Competing Interests: None
Funding: The authors received no specific funding for this work.
References
- Bäck H, Gustavsson B, Ridell B, Rödjer S, Westin J. Primary gastrointestinal lymphoma incidence, clinical presentation, and surgical approach. J Surg Oncol, 1986; 33(4): 234–238.
- Ghai S, Pattison J, Ghai S, O’Malley ME, Khalili K, Stephens M. Primary Gastrointestinal Lymphoma: Spectrum of Imaging Findings with Pathologic Correlation. RadioGraphics, 2007; 27(5): 1371–1388.
- Ghimire P. Primary gastrointestinal lymphoma. World J Gastroenterol, 2011; 17(6): 697.
- Smith C, Kubicka RA, Thomas CR. Non-Hodgkin lymphoma of the gastrointestinal tract. RadioGraphics, 1992; 12(5): 887–899.
- Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology (Phila), 2018; 50(1): 74–87.
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IB de O, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia, 2022; 36(7): 1720–1748.
- Martelli M, Ferreri AJM, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol, 2013; 87(2): 146–171.
- Karna R, Mohy-Ud-Din N, Zhang Q, Babich M. S2051 Primary Esophageal Lymphoma or Lymphoma Presenting as an Esophageal Mass: Rare Disease or Rare Presentation of a Disease? Am J Gastroenterol, 2021; 116(1): S888–S889.
- Sanchez-Bueno F, Garcia-Marcilla JA, Alonso JD, Acosta J, Carrasco L, Piñero A, et al. Prognostic factors in primary gastrointestinal non-Hodgkin’s lymphoma: a multivariate analysis of 76 cases. Eur J Surg, 2003; 164(5): 385–392.
- Ogura T, Tajika M, Hijioka S, Hara K, Haba S, Hosoda W, et al. First report of a mucosa-associated lymphoid tissue (MALT) lymphoma of the esophagus diagnosed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). Endoscopy, 2012; 44(S 02): E167–E168.
- Peng JC, Zhong L, Ran ZH. Primary lymphomas in the gastrointestinal tract: Primary GI lymphomas. J Dig Dis, 2015; 16(4): 169–176.
- Gu J, Chan T, Zhang J, Leung AYH, Kwong YL, Khong PL. Whole-Body Diffusion-Weighted Imaging: The Added Value to Whole-Body MRI at Initial Diagnosis of Lymphoma. Am J Roentgenol, 2011; 197(3): W384–W391.
- O’Malley DP, Auerbach A, Weiss LM. Practical Applications in Immunohistochemistry: Evaluation of Diffuse Large B-Cell Lymphoma and Related Large B-Cell Lymphomas. Arch Pathol Lab Med, 2015; 139(9): 1094–1107.
- Lino-Silva LS, Salcedo-Hernández RA, Molina-Frías E, Padilla-Rosciano A, Avilés-Salas A. Primary esophageal CD30-positive ALK-positive anaplastic large cell lymphoma with MUM1 expression. Esophagus, 2013; 10(1): 51–54.
- Sharma B, Pavelock N, Antoine M, Shah M, Galbraith K, Rawlins S. Primary Diffuse Large B-Cell Lymphoma of the Descending Colon. Am J Med Sci, 2019; 358(2): 164–167.
- Chadha KS, Hernandez-Ilizaliturri FJ, Javle M. Primary Esophageal Lymphoma: Case Series and Review of the Literature. Dig Dis Sci, 2006; 51(1): 77–83.
- Bartlett DL, Karpeh MS, Filippa DA, Brennan MF. Long-Term Follow-Up After Curative Surgery for Early Gastric Lymphoma: Ann Surg, 1996; 223(1): 53–62.

