Identifying the Toxidrome of Ivermectin Toxicity
Jon Stewart H Dy* and Dan Neftalie Juangco
Institute for Neurosciences, St. Luke’s Medical Center, Quezon City, Philippines
Received Date: 03/07/2023; Published Date: 21/11/2023
*Corresponding author: Jon Stewart H. Dy, MD, Institute for Neurosciences, St. Luke’s Medical Center – Quezon City, E. Rodriguez Sr. Avenue, Quezon City, Metro Manila, Philippines
Abstract
Ivermectin is an antiparasitic drug that has been used as an alternative for prophylaxis and treatment of COVID-19 infection. The adverse effects from supratherapeutic doses of Ivermectin can include non-neurological and neurological symptoms. In this study, we report the case of a 52-year-old Filipino male with newly diagnosed diabetes mellitus who developed a subacute history of fever, cough and generalized weakness, causing him to self-medicate with supratherapeutic doses of Ivermectin and thereafter subsequently developed decrease in sensorium, restlessness and complex visual hallucinations. Significant laboratory examinations showed hyperglycemia, mild hyponatremia, positive SARS-CoV2 reverse transcriptase polymerase chain reaction test and bilateral pneumonia on chest radiograph. He was subsequently started on antibiotics, high flow nasal cannula and given two doses of activated charcoal. During the first 24 hours of hospital admission, there was significant improvement in the patient’s sensorium with resolution of restlessness and visual hallucinations. During the rest of the hospitalization, his respiratory symptoms had improved and he was subsequently discharged. Clinical outcome in our patient after administration of activated charcoal and completion of antibiotics showed an overall improvement in symptoms and without any neurologic sequelae.
Introduction
Ivermectin is a derivative of avermectins and an antiparasitic drug that acts mainly by binding to glutamate-gated chloride channels, causing an increase in membrane permeability that leads to neuromuscular paralysis and parasite death [1]. Reports from in vitro studies have suggested that Ivermectin can interfere with the attachment of SARS-CoV-2 spike protein to the human cell membrane, inhibition of host transport proteins that the virus uses to evade the antiviral response, and some studies suggesting anti-inflammatory properties [2]. Despite this proposed clinical benefit, its use is limited to several tropical diseases such as scabies, helminthiases and onchocerciasis. It is not approved by the Food and Drug Administration (FDA) for the treatment of any viral infection [1,2]. At present, there is insufficient evidence to recommend or discourage the use of Ivermectin for the prevention and treatment of COVID-19 infection. Although the drug is generally well tolerated, non- neurological adverse effects may include nausea, vomiting, diarrhea, pruritus and dizziness while neurological adverse effects may include headache, encephalopathy, confusion, stupor, coma, dizziness, vertigo and tremor [3,4]. There are no available data on the therapeutic window of Ivermectin. A case report shows that those with human ATP-binding cassette subfamily B member 1 (ABCB1) transporter nonsense mutations are those at risk for developing toxicity even with intake of therapeutic doses of Ivermectin [4]. Although studies on the neurological adverse effects of Ivermectin are not locally available in the Philippines, a pharmacovigilance case series study showed serious adverse reactions such as toxidermias, encephalopathies and confusional disorders after Ivermectin exposure [3]. In this study, we report the first locally-documented case of Ivermectin overdose after intake of more than 10x the recommended maximum therapeutic dose of Ivermectin.
Case Report
This patient is E.R., a 52-year-old male from the Philippines, with a significant medical history of newly-diagnosed diabetes mellitus (glycohemoglobin level = 13.2%). He presented with a chief complaint of decrease in sensorium. He works as a dentist and is independent on all activities of daily living. He is not on any maintenance medications and he has no other known comorbid illnesses. He has poor health-seeking behavior and he completed his primary doses of AstraZeneca COVID-19 vaccination 1.5 months prior to hospital admission at St. Luke’s Medical Center. He had no other known vices such as smoking and alcoholism.
On August 13, 2021, he started experiencing high-grade fever, nonproductive cough, generalized body weakness and decrease in appetite, for which febrile episodes would resolve with as needed antipyretics. Interim history showed persistence of symptoms. On August 26, 2021, because of persistence of symptoms, he self-medicated with 108 mg of Ivermectin (12mg per tablet, total of 9 doses given) with no noted improvement of symptoms. On August 27, 2021, his symptoms had further worsened, he again self-medicated with 216 mg of Ivermectin (12mg per tablet, total of 18 doses given, cumulative dose of 4mg/kg taken in a 24-hour time period), and approximately 5 hours after intake, he then became drowsy, restless, agitated, confused and had experienced complex visual hallucinations, tremulousness and gait instability. These symptoms eventually prompted hospital admission. On assessment, his systemic physical examination was significant for high oxygen requirement (on high flow nasal cannula with flow rate 30 liters per minute, FiO2 requirement of 100%), stable other vital signs, no signs of cardiorespiratory distress, a clinically hypovolemic state, and coarse bilateral crackles. The rest of his systemic physical examination was unremarkable. There were no noted skin or oral lesions, rashes, pallor, jaundice or organomegaly. Neurological examination showed nonsustained eye opening to vigorous stimulation, inconsistent regard, unable to follow commands, with psychomotor agitation, pupils 3mm isocoric and briskly reactive to light, midline gaze, roving eye movements, no facial asymmetry, limited verbal output with no noted involuntary movements, with spontaneous and symmetric movement of all extremities. He was able to localize to pain on the bilateral upper extremities and withdraw to pain on the bilateral lower extremities, with supple neck, no pathologic reflexes while full cerebellar and gait examination were difficult to assess given the level of consciousness. The differential diagnosis on admission included toxic-metabolic encephalopathy versus COVID-related septic and hypoxic encephalopathy, to rule out a CNS infection (viral encephalitis) and nonconvulsive seizures.
Laboratory examinations (Table 1) showed hyperglycemia, mild hyponatremia, hyperkalemia, elevated inflammatory markers, mildly elevated creatinine kinase, normal hemoglobin and hematocrit, normal white blood cell count and platelet count, normal kidney and thyroid function, normal bilirubin and liver enzymes, normal bleeding parameters, ketonuria, normal sinus rhythm on electrocardiogram, trace serum ketones, normal acid base status on arterial blood gas, positive SARS-CoV2 reverse transcriptase polymerase chain reaction test, and bilateral middle to lower lung pneumonia on chest radiograph. No available serum nor urine assay to measure Ivermectin was available. Cranial magnetic resonance imaging with contrast showed unremarkable findings (Figure 1). A 2-hour video electroencephalogram (Table 2) showed mild to moderate diffuse cerebral dysfunction and absence of seizure activity. Patient was admitted to the progressive care unit, maintained on high flow nasal cannula and nasogastric tube, administered with Ceftriaxone 2 grams and Azithromycin 500mg intravenously. He was then given two doses of activated charcoal (total of 100mg) and laxatives. A CSF analysis was no longer done since his neurologic status had significantly improved within 24 hours after initiation of treatment, where he was now noted to be awake, alert, oriented, able to follow commands, with no other focal neurologic deficits. He was then subsequently managed as a case of multifactorial encephalopathy secondary to COVID-19 infection and Ivermectin toxicity. By the second hospital day, patient’s neurological symptoms had completely resolved, antibiotics were continued and he was gradually weaned off oxygen support. He was then discharged stable after eight hospital days. Clinical outcome on follow-up of the patient shows no residual symptoms and neurologic deficits, and he was able to resume his normal daily activities thereafter.
Table 1: Serum tests performed during the course of the hospital admission.
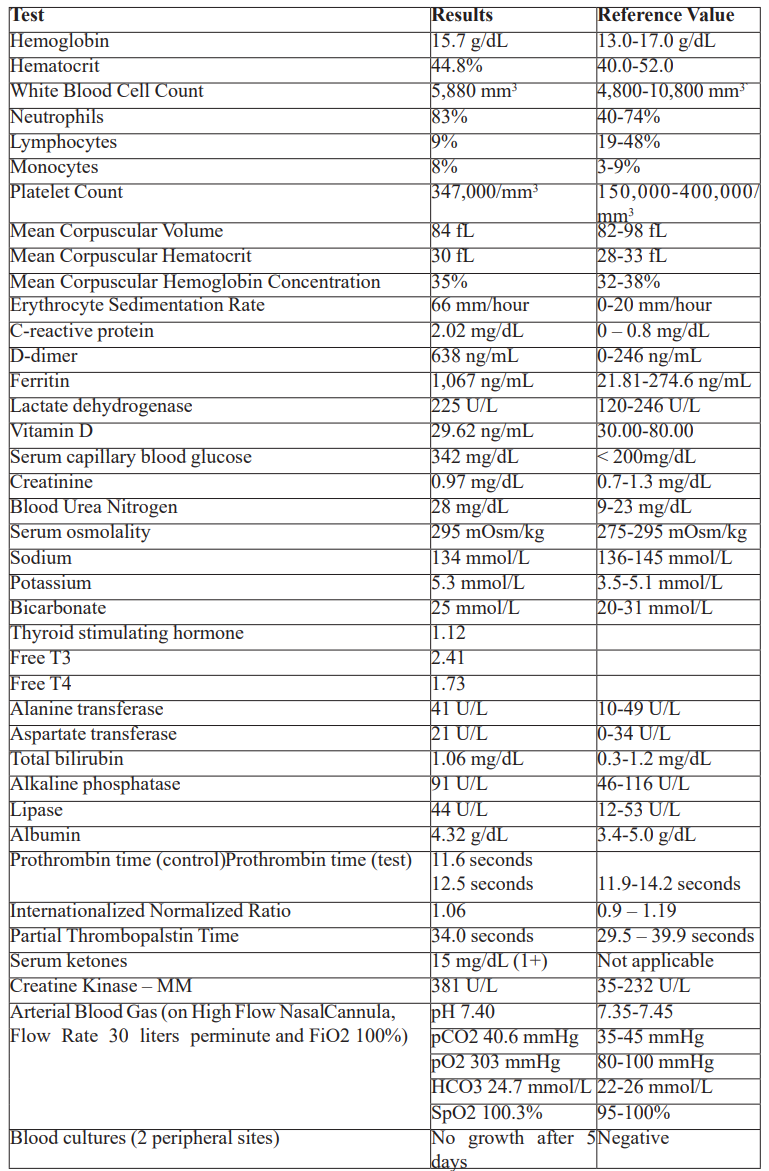
Table 2: 2-hour video electroencephalogram results.
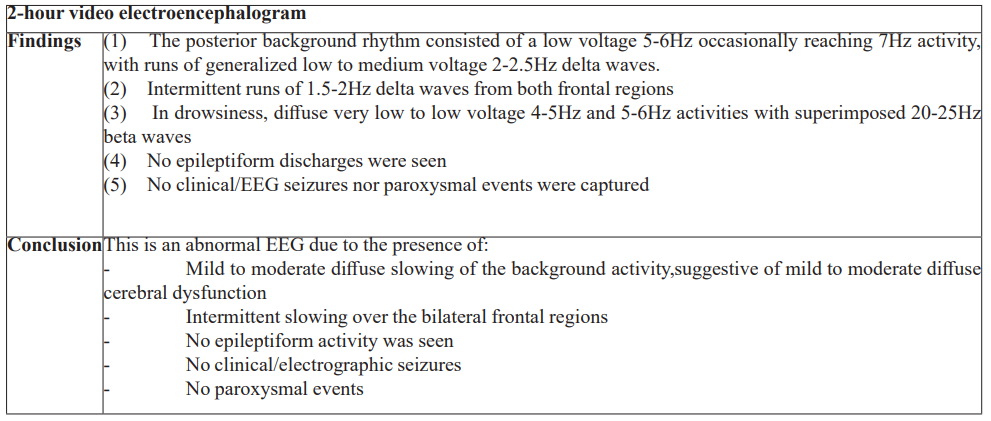
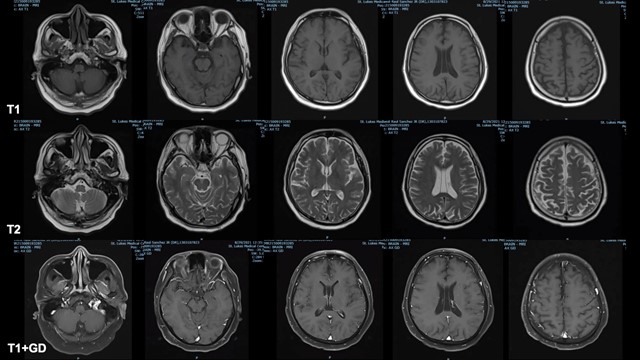
Figure 1. Cranial magnetic resonance imaging with contrast results. Top row: Axial T1 Representative Images; Middle Row: Axial T2 Representative Images; Bottom Row: Axial T1 with Contrast Representative Images.
Discussion
Since the start of the COVID-19 pandemic, the use of Ivermectin in the prevention and treatment of SARS-CoV2 infection has gained widespread popularity. In vitro studies suggest that Ivermectin acts by inhibiting the host importin alpha/beta-1 nuclear transport proteins, which are part of an intracellular transport process that viruses hijack to enhance infection by suppressing the host’s antiviral response, underlying its use against viruses such as dengue virus, yellow fever virus and Zika virus. Studies have also suggested anti-inflammatory properties, which have been hypothesized to be beneficial in SARS-CoV2 infections [3-5]. Despite these studies, no clinical trials have reported a clinical benefit of Ivermectin against all these viral infections. Additionally, the dose required to achieve the adequate plasma concentrations necessary for antiviral efficacy would require those up to 100-fold higher than the dose approved for use in humans [4,7].
At therapeutic doses, Ivermectin does not readily penetrate the blood-brain barrier, where GABA functions as a neurotransmitter. The therapeutic dosage recommended is 0.2-0.3mg/kg/day with a maximum daily dosage of 0.4mg/kg/day [3,4]. Ivermectin has a high margin of safety in humans and does not readily cause serious adverse drug reactions since it is transported out of the central nervous system by ATP-binding cassette subfamily B member 1 (ABCB1) transporter [3]. It has also been considered to be free of the potential to cause neurological adverse drug reactions due to the low drug levels in the central nervous system, except in situations of drug overdose [1,2,5]. Neurological adverse effects have been reported in those treated for parasitic diseases, in doses above 2mg/kg/day [8,9]. Symptoms of neurotoxicity include lethargy, drooling, tremors/seizures, gait imbalance, decrease in sensorium and disorientation [4]. In patients with mutations of the ABCB1 transporter, intake of therapeutic doses of Ivermectin will result in serious neurological symptoms such as coma, ataxia, pyramidal signs and binocular diplopia [3,7]. Non-neurological adverse events included pruritus, myalgia, cough, difficulty of breathing, nausea and vomiting, diarrhea, postural hypotension and serious skin reactions and edematous swelling [8,9].
At present, there are no local case reports in the Philippines on the serious adverse drug reactions of Ivermectin in patients with SARS-CoV2 infection. There are also no guidelines that exist for the treatment of Ivermectin toxicity and there is no specific antidote for Ivermectin overdose and management remains to be supportive in cases of overdose. The benefit of administering activated charcoal in alleviating the adverse drug effects of Ivermectin overdose is probably due to the accelerated fecal route of drug elimination, as was seen in our patient’s case [1].
In the context of the pandemic and the continued use of Ivermectin for the prophylaxis and treatment of SARS-CoV2 infection, it is difficult to conclude that a patient’s neurological symptoms are solely associated with Ivermectin toxicity as many patients who develop severe SARS-CoV2 infection will develop similar symptoms [6]. The American Association of Poison Control Center has reported similar adverse drug reactions from the use of Ivermectin, for which most of the cases reported had minimal clinical effects and its use was intended for prophylaxis or treatment of SARS-CoV2 infection. Locally, the University of the Philippines National Poison Management and Control Center has reported a total of 16 cases of Ivermectin exposures, all of which had consumed the recommended daily dose of Ivermectin. Most of the reported cases were unintentional, with none reporting neurological symptoms similar to our index case [10]. It is therefore essential for clinicians to properly educate patients on the use of Ivermectin and that it has unproven benefits in the prophylaxis and treatment of SARS-CoV2 infection.
Conclusion
In summary, we present the case of a 52-year-old Filipino male who was treated as a case of multifactorial encephalopathy secondary to Ivermectin toxicity and SARS-CoV2 infection in the Philippines. Any serious non-neurologic and neurologic adverse effects must be reported to educate the public on the risks of unintentional and intentional use of Ivermectin. Despite the lack of treatment guidelines for cases of Ivermectin overdose, activated charcoal has shown to be efficacious in our patient’s case and should be considered as a therapeutic option. While the clinical syndrome of Ivermectin toxicity has yet to be fully identified, further studies are needed to identify all the possible short-term and long-term complications with the use of Ivermectin. Therefore, it is important for clinicians to recognize the adverse drug effects associated with the use of Ivermectin. Lastly, it is important to highlight Ivermectin has uncertain health benefits in the prevention and treatment of SARS-CoV2 infection.
Acknowledgements
We would like to thank Dr. Dan Neftalie Juangco for the index case, manuscript formatting and proofreading of the article. We would like to thank Dr. Carissa Dioquino for the statistics from the University of the Philippines National Poison Management Control Center.
Disclosures
Human subjects: Consent was obtained by all participants in this study.
Conflicts of interest: None of the authors have any conflicts of interest to disclose.
Payment/services information: All authors have declared that no financial support was received from any organization for the submitted work.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- Katzung BG, Masters SB, Trevor AJ. Basic & clinical McGraw-Hill, United States of America. 2021; 52: 915-936.
- Kinobe RT, Owens L. A systematic review of experimental evidence for antiviral effects of ivermectin and an in-silico analysis of ivermectin's possible mode of action against SARS-CoV-2. Fundamental & Clinical Pharmacology, 2021; 35: 260-276. 1111/fcp.12644
- Kanza EM, Nyathirombo A, Larbelee JP, et al. O. volvulus microfilariae in the anterior chambers of the eye and ocular adverse events after a single dose of 8 mg moxidectin or 150 µg/kg ivermectin: Results of a randomized double-blind Phase 3 trial in the Democratic Republic of the Congo, Ghana and Liberia, 2023; 1-26. 21203/rs.3.rs-2879768/v1
- Baudou E, Lespine A, Durrieu G, et al.: Serious ivermectin toxicity and human ABCB1 nonsense New England Journal of Medicine, 2020; 383: 787-789. 10.1056/NEJMc1917344
- Chandler RE. Serious neurological adverse events after ivermectin—do they occur beyond the indication of onchocerciasis? The American journal of tropical medicine and hygiene, 2018; 98: 382. 4269/ajtmh.17-0042
- Ropper A, Samuels M, Klein J, et al. Adams and Victor’s Principles of Neurology. McGraw-Hill, United States of America; 41:1209-1250.
- COVID-19 Clinical Features, 2020.
- Edwards G. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria Journal, 2003; 2: 1-6. 1186/1475-2883-2-S1-S8
- Campillo JT, Boussinesq M, Bertout S, et Serious adverse reactions associated with ivermectin: A systematic pharmacovigilance study in sub-Saharan Africa and in the rest of the World, 2021; 15: 1-18. 10.1371/journal.pntd.0009354
- National Poison Control and Information Service,

