Lung Consolidation in a Young-Age Active Smoker: An Unexpected Diagnosis
Bertuccio FR*, Baio N, Chino V, Ferroni V, MontiniS, Pisanu L, Sanci V, Cascina A, Conio V and Corsico AG
Cardiothoracic and Vascular Department, Unit of Respiratory Disease, IRCCS Policlinico San Matteo-Viale Golgi 19, Italy; Department of Internal Medicine and Pharmacology, University of Pavia, Italy
Received Date: 21/06/2023; Published Date: 11/11/2023
*Corresponding author: Bertuccio FR, Cardiothoracic and Vascular Department, Unit of Respiratory Disease, IRCCS Policlinico San Matteo- Viale Golgi 19, 27100 Pavia, Italy; Department of Internal Medicine and Pharmacology, University of Pavia, Via Lombroso 17, Pavia 27100, Italy
Abstract
A 29-year-old man presented to Emergency Department with chest pain and non-productive-cough accompanied by by night sweat and weight loss. Through a series of radiological and invasive diagnostic studies we finally reach an unexpected diagnosis of hypercreativity pneumonitis; this is a complex and heterogeneous disease which diagnosis can be challenging as its clinical, radiologic and histopathologic features overlap with those of other Interstitial Lung Diseases (ILDs) and it may not be possible to identify a culprit exposure. Diagnosing an ILD is a dynamic process, and that is the reason why complex cases discussed in a multidisciplinary team may need to be reconsidered in light of evolution of the disease and the results of the performed exams with a flexible approach.
Keywords: Hypersensitivity pneumonitis; Interstitial lung diseases; Multidisciplinary-team discussion
Introduction
Hypersensitivity Pneumonitis (HP) is a complex and heterogeneous disease. Making diagnosis can be challenging as its clinical, radiologic and histopathologic features overlap with those of other Interstitial Lung Diseases (ILDs). In this case report we highlight the importance of multidisciplinary team discussion especially in most misleading situation.
History
A 29-year-old man presented to Emergency Department with chest pain and non-productive-cough. His symptoms had been ongoing for 2 months and were accompanied by night sweat, weight loss (approximately 5 kg in 3 months), but no fever. He was previously a fit and well warehouse worker with no past medical history. He was not taking any regular medications and there was no known allergy. He was an active smoker of 20 cigarettes/day for 4 years and admitted to previous cannabinoids use. He denied any family history for respiratory disease. He did admit to an ongoing mould exposure in his dwelling. An initial chest x-ray showed evidence of para-hilar left upper lobe consolidation with indistinct margins, compatible with inflammatory thickening. He was sent home for a presumed respiratory tract infection with antibiotic therapy of levofloxacin with a plan for follow-up Interval chest x-ray. After 7 days the symptoms did not resolve and follow-up chest x-ray after antibiotic therapy was unchanged, so we proceeded to a thorax CT scan with IV contrast. The CT (Figure 1) showed a marked progression of number, density and dimension of the already present cystic alteration on previous HRTC scan, with evidence of consolidation in the anterior segment of the left upper lobe. He was therefore referred to our respiratory team for further inpatients work-up.
Physical examination and findings:
On admission physical examination the patient appeared in no acute distress. Vital signs revealed a temperature of 36.5°C, peripheral pulse of 68 beats/minute, respiratory rate of 16/min and blood pressure of 130/80 mmHg and oxygen saturation of 98% on room air. Respiratory examination revealed scattered bilateral crackles in absence of wheezes or rubs. Cardiac and abdominal examinations were unremarkable. Laboratory examination of peripheral blood highlighted a borderline increase in inflammatory markers: C-reactive protein was 0,75 mg/dL and procalcitonin was negative; the remaining admission blood exams were unremarkable.
Diagnostic studies:
Following up from the CT thorax findings, further imaging was arranged as an impatient in the form of PET scan (Figure 2). There was significant uptake from the opacification in the left upper lobe (SUV 13.83) and from mediastinal lymph nodes in the aortopulmonary region (SUV 4.46), paratracheal (SUV 4), sub-carinal (SUV 5.34) e hilar regions bilaterally (SUV 4.82). PET alterations were suggestive for lymphoproliferative disease or a granulomatous disease. Consequently, a lung tissue biopsy of the apical portion of the left lung in video assisted thoracoscopy was arranged. The histological exam evidenced: inflammatory reactive infiltrate characterised by giant cell foreign body-like cellular components and an exuberant reaction identifying an inflammatory pseudotumor. The cultural tests and research were all negative. In addition, the patient underwent an endobronchial ultrasound with transbronchial needle aspiration (EBUS TBNA) in order to biopsy mediastinal lymph nodes which was not conclusive, highlighting lymphocytes aggregation with some bronchial epithelial cells. Contextually to EBUS a bronchoalveolar lavage (BAL) was performed; the cultures from the lavage were negative and tuberculosis infection was again excluded. Another HRTC Thorax scan was performed with evidence of new appearance of opacities in the right lung.
Diagnosis:
The case was therefore discussed with a Multidisciplinary Team (MDT) composed of respiratory physician, thoracic surgeon, radiologist and pathologist; the MDT agreed the next step would be a lung segment biopsy during Video Assisted Thoracoscopy (VATS). This time the histology exam was conclusive for Hypersensitivity Pneumonitis (HP). In hindsight the upper lung lobes localisation of disease, the referred exposure to moulds and the chest CT scan lesions’ tendency to evolve, all support the diagnostic hypothesis of an unusual form of hypersensitivity pneumonitis.
Clinical Course
Six months of systemic corticosteroid therapy was completed with gradual resolution of symptoms. Three months after the discontinuation of the treatment, a follow-up HRCT scan (Figure 3) showed complete resolution of the opacification in the left upper lobe and in the right lung. The cystic alterations and the bilateral hilar lymphadenopathy were stable. There was no evidence of new appearance of ground-glass or solid lesions. At the time of writing, the patient remains under our outpatient follow-up and had no recurrence of disease.
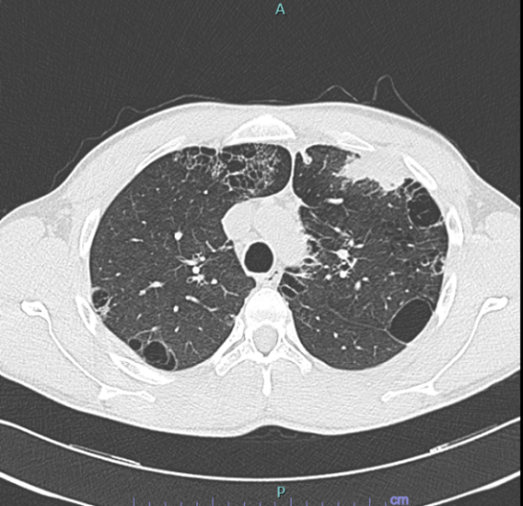
Figure 1
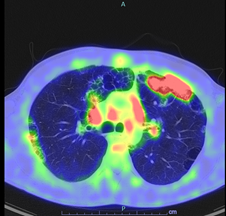
Figure 2
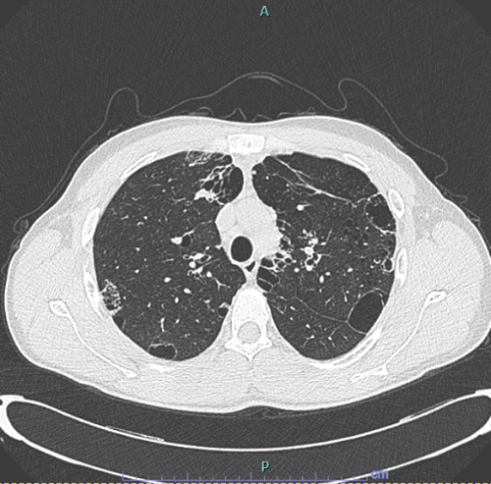
Figure 3
Discussion
HP is a complex and heterogeneous disease. Making a diagnosis of HP can be challenging as its clinical, radiologic and histopathologic features overlap with those of other interstitial lung diseases (ILDs) and it may not be possible to identify a culprit exposure [1].
Differential diagnosis mainly concerns with radiologic (fibrosing and not fibrosing) and histopathologic datas:
For what concerns with histopathology: biopsy should be avoided in patients in whom a confident diagnosis of HP can be made with the available datas. However sometimes, obtaining lung tissue is recommended if warranted based on the risk/benefit for the individual patient. Tissue samples should be reviewed by a pathologist experienced in ILD in the context of clinical and radiologic informations [2,3]. The usefulness of surgical lung biopsy for the diagnosis of ILD remains controversial, since they are associated with a high morbidity and mortality. VATS is generally considered to be a safer procedure that provides lung tissue samples that are sufficient for a definitive histopathological diagnosis. Often, biopsy is deemed impracticable due to age, disease severity, comorbidities, immunocompromised status, or hypoxemic respiratory failure. In our case description, the patient has no relevant comorbidities and considering the young-age has an optimal performance status; consequently, surgical diagnostic tools proved to be conclusive [3].
Managing with patients affected by ILD means to deal with some diagnostic uncertainty and yet have to take diagnostic decisions to best advise the patients about how to manage their disease. Multiple factors can be helpful in this. Above all, multidisciplinary decisions increase the accuracy of the diagnosis and allow it to be based on the consensus of experts rather than on the opinion of individual physicians. MDT has become the gold standard for the diagnosis of many ILD yet.
Conclusions
-The diagnostic approach to HP has evolved as our knowledge of ILD is improving but still remains complicated. The currently approved diagnostic criteria depend on a series of findings about the patient and are still under discussion.
- Both surgical and non-surgical biopsy techniques have experienced progress with regards to safety and diagnostic yield in ILD. Further research, continued monitoring, robust patient selection processes and multidisciplinary shared decision-making are some ways to optimise outcomes whilst minimizing risk.
-Diagnosing an ILD is a dynamic process, and that is the reason why complex cases discussed in a multidisciplinary team may need to be reconsidered in light of evolution of the disease and the results of the performed exams, with a flexible approach. Nowadays diagnostic guidelines better correspond to the pragmatic management adapted to real practice and with our case description we want to put paramount importance around the Multidisciplinary discussion and its workup.
Acknowledgements:
Guarantor: F.R.Bertuccio had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
N.Baio, V.Chino, V.Ferroni, S.Montini, L.Pisanu, V.Sanci, A.Cascina, V.Conio, A.G.Corsico contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript
Competing interest: All authors declare that they have no conflicts of interest.
Grant information: The authors received no specific funding for this work.
References
- Andrea L Magee*, Steven M Montner, Aliya Husain, Ayodeji Adegunsoye, Rekha Vij, Jonathan H Chung. Imaging of Hypersensitivity Pneumonitis. Published in final edited form as: Radiol Clin North Am, 2016; 54(6): 1033–1046. doi: 10.1016/j.rcl.2016.05.013.
- David A Lynch, MB Denver. CT Phenotypes in Hypersensitivity Pneumonitis. DOI https://doi.org/10.1016/j.chest.2018.10.048
- Hamblin M, Prosch H, Vašáková M. Diagnosis, course and management of hypersensitivity pneumonitis. Eur Respir Rev, 2022; 31: 210169. DOI: 10.1183/16000617.0169-2021.

