A Case Report and Literature Review of Low-Grade Fibrocytic Sarcoma of the Soft Tissue of the Knee Joint
Tao Cheng1,2, Shaohua Liang2, Jinli Zhang3 and Wen Wang1,2,*
1Department of Clinical Medicine, Guizhou Medical University, Guiyang, Guizhou 550000, China
2Department of Orthopaedics, Guangzhou Red Cross Hospital, Guangzhou, Guangdong 510220, China
3Department of Orthopedics, Guangzhou Institute of Traumatic Surgery, Guangzhou Red Cross Hospital, China
Received Date: 13/06/2023; Published Date: 01/11/2023
*Corresponding author: Wen Wang, Department of Clinical Medicine, Guizhou Medical University, Guiyang, Guizhou, Department of Orthopaedics, Guangzhou Red Cross Hospital, Guangzhou, Guangdong, 510220, China
Abstract
We report a low-grade malignant myofibrocytic sarcoma of the soft tissue in the knee joint. The patient presented at clinic for finding a soft tissue mass on the medial side of the left knee joint. In the surgery, a yellow-white mass above the sartorius muscle was found which was clear boundary with surrounding tissues. We completely excised the tumor and postoperative pathology revealed low-grade malignant myofibroblastic sarcoma. Our patient's quality of life was improving and without recurrency with 1 year followed-up.
Keywords: Low-grade malignant fibrocytic sarcoma; Soft tissue of knee joint; Resection
Introduction
Low-Grade Myofibroblastic Sarcoma (LGMS) is an atypical myofibroblastic tumor with fibromatosis-like features that most commonly occurs in male adults, usually in the head and neck [1]. LGMS is uncommon in clinical cases, and there are few reported cases. LGMS in most parts are ignored in the early stage because of the painless mass, or misdiagnosed as other diseases with similar symptoms or images [11,14], Therefore, the diagnosis of LGMS is extremely difficult. LGMS has been reported in the scalp, jaw, oral cavity, levator scapula, pancreas, and lower femur [2-10]. Knee LGMS is rarely reported. We report a 75-year-old female patient with knee LGMS and review the literature.
Case Report
A 75-year-old female patient had an acute course of disease. The patient presented to the hospital 15 days after discovering a mass on the medial and posterior side of the left knee. On admission, the physical examination revealed that the mass was soft and movable, with clear boundaries with surrounding tissues and no tenderness. MR showed a tumor at the posterior edge of the left knee sartorius muscle, with a size of about 3.9*1.9 cm. T2WI showed low mixed signal, T1WI showed low signal, and T2WI fat suppression sequence showed high and low mixed signal. It was obviously enhanced after enhancement, and the source of fibrous tumors was considered. After admission, preoperative examinations were completed and tumor resection was performed. During the operation, a tough yellow-white mass was found above the sartorius muscle of the left knee. There was no adhesion to the surrounding tissues. The tumor had clear boundaries, a complete capsule, and a non-invasive growth. After the tumor was carefully separated from the surrounding tissue, the tumor was completely excised and examined pathologically. The pathological report showed that the tumor cells were spindle-shaped or polygonal, arranged in bundles, and partially arranged in a herringbone-like arrangement. The cells showed mild to moderate atypia. Tumor giant cells and odd-shaped cells could be seen, and mitotic figures were visible but not clearly seen. Tumor necrosis, interstitial see chronic inflammatory cell infiltration. The immunohistochemistry results included Vimentin(+), CD99(+), Actin fraction (+), β-catenin fractional nucleus (+), Ki67 hotspot 15% (+), residual immunohistochemistry CD68(-), SMA(-), Desmin (-S100(-), CK(-), Lysozyme (-), P63(-), CD10(-), CD34(-), ALK(-), Calp onin(-). The medial knee mass is an interlobar spindle cell tumor, which is diagnosed as low-grade malignant myofibroblastic sarcoma combined with pathological and immunohistochemical results. After the operation, the patient was discharged from the hospital smoothly, and the outpatient clinic had regular follow-up visits. After the wound healed, he returned to normal life, and the joint function was satisfactory. There was no recurrence after 1 year of follow-up.
Discussion
LGMS was first proposed by Vasude et al. in 1978, which was confirmed by Mentzel et al. in 1998, and finally confirmed by Montgomery et al. as a unique entity [8]. Due to the lack of unified diagnostic criteria, LGMS was also called myofibrosarcoma, myofibroblast sarcoma, myofibroblast-rich fibrosarcoma, and leiomyosarcoma in the past [13]. LGMS was first classified as a distinct entity in the 2002 WHO Classification of Pathology and Genetics of Tumors of Soft Tissue and Bone. In subsequent editions, it is still referred to as LGMS and is classified as part of the fibroblastic/myofibroblastic tumor category. According to literature statistics, LGMS occurs frequently in the head, neck and extremities, and is prone to local recurrence, and the oral cavity (especially the tongue) is the most common affected area [7,17]. So far, LGMS is uncommon in clinical cases, and few cases have been reported. Mainly due to the lack of unique biological characteristics of LGMS and the lack of in-depth imaging studies, the pathological identification of myofibroblasts is mainly diagnosed by light microscopy and immunohistochemistry [8,10]. Zhang Shikun [3] et al. mentioned in the literature that in vivo molecular imaging had unique advantages in diagnosing tumors. It can obtain higher spatial resolution at a lower cost and can detect highly sensitive, high-resolution imaging in deep tissues. Therefore, it is speculated that the new molecular imaging technology may be helpful for the diagnosis and prognosis of LGMS. LGMS in most parts is ignored in the early stage because of the painless mass, or misdiagnosed as other diseases with similar symptoms or images [11,14], most often misdiagnosed as inflammatory myofibroblastic tumor (IMT). Both LGMS and IMT belong to myofibroblastic tumors, which are similar in tissue morphology and MR appearance, and are easily confused [3,13]. However, there are differences in growth characteristics, morphology, and immunohistochemistry between the two diseases. This is similar to the analogous report in the previous article. The patient had no specific symptoms and abnormal physical examination in the outpatient physical examination, and the palpation of the tumor was similar to that of a conventional lipoma. Ultrasound and MRI examination reports indicated that abnormal booth lesions were admitted to hospital for treatment. During the operation, the shape of the tumor was obviously different from that of conventional lipoma, and the general shape of the specimen did not have typical characteristics. Mana [16] found that LGMS were loose, branched fragments of spindle-shaped tumor cells with unclear cell boundaries during the needle aspiration cytology detection of LGMS. Nuclei vary in shape from ovate to fusiform, including angular and bare nuclei. Nuclei are mildly to moderately pleomorphic, with occasional nucleoli. Ni C [12] et al. concluded in the literature that plasma cell inflammatory cell infiltration is obvious in IMT, but rare in LGMS; LGMS has a more uniform histological pattern, with more mitotic figures and nuclear atypia; in Cytokeratin and ALK are present in some subpopulations of IMTs but absent in LGMS. Combining several factors can help to make a differential diagnosis between the two diseases.
For the current treatment of LGMS, a unified plan has not been reached in the existing cases. The current optimal treatment plan for LGMS is still controversial, and there are large differences in efficacy. However, surgical treatment is still the main method, and a small number of patients use surgery plus radiotherapy and chemotherapy [6,10]. Long Peng [6] et al. reported a case of pancreatic LGMS after two cycles of adjuvant chemotherapy for 5 years without tumor recurrence; Niu Rong [15] et al. reported a case of gastric cardia LGMS that passed away 3 years later due to tumor progression without undergoing postoperative radiotherapy and chemotherapy. Tessho Maruyama [17] found that the recurrence rate of LGMS patients after surgery alone was 18.8%, while the recurrence rate after surgery combined with radiotherapy was 71.4%. This suggested that postoperative radiotherapy for LGMS may contribute to tumor recurrence, and it was recommended to avoid radiotherapy after LGMS resection. Ni [12] et al. suggested that the treatment of LGMS requires extensive resection, confirming that there was no residual tumor in the margin of resection, and performing postoperative radiotherapy and chemotherapy if necessary. Therefore, the treatment method should be selected according to the tumor location, size, and degree of invasion. Complete intraoperative resection was used in this case, and there was no sign of recurrence after 1 year of outpatient follow-up.

Figure 1: Preoperative Magnetic Resonance Imaging (MRI). MRI showed a mass in the sartorius muscle of the knee joint. The mass was 3.9×1.9 cm in size, with clear borders, low mixed signal on T2WI, low signal on T1WI, high and low mixed signal on T2WI fat-suppressed sequence, and obvious enhancement after enhancement. The tumor was considered to be of fibrous origin.

Figure 2: Re-examination after resection of the sartorius soft tissue mass in the left knee joint. Irregular strips of long T1 and slightly shorter mixed T2 signal shadows were seen in the operation area, which adhered to the surface of the adjacent sartorius muscle. After enhancement, there was slight enhancement, but no part was seen Definite soft tissue mass.
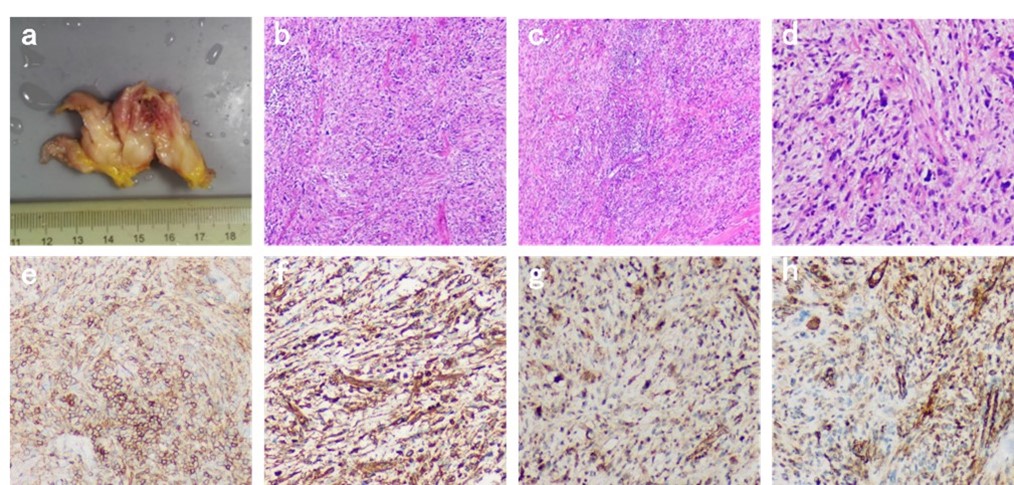
Figure 3: a: Postoperative gross photos: Irregular gray white, gray yellow tissue can be seen with the naked eye, with a total volume of 7x4x1.5cm. Some tissue sections are gray white, with a slightly tough texture; b. C, d: Under light microscopy, tumor cells in the tissue are composed of spindle or polygonal shapes, with tumor giant cells and bizarre cells visible, mitotic figures visible, no clear tumor necrosis observed, and chronic inflammatory cell infiltration seen in the stroma; e: CD99 (+), f: Vimentin (+) g: β- Catenin partial core (+) h: Action partial core (+).
Conclusion
In summary, LGMS is still a rare malignant tumor, prone to local recurrence and rare in distant metastasis. In this case, we report a rare case of LGMS of the knee with an intact capsule and a short course. There was no sign of recurrence 1 year after the operation. Given the possibility of recurrence, long-term follow-up of patients is crucial.
References
- Hirotaka Yonezawa, et al. Low-grade myofibroblastic sarcoma of the levator scapulae muscle: a case report and literature review. BMC Musculoskeletal Disorders, 2020; 21(1): 1-8.
- Han Seong Rok and Yee Gi Taek. Low Grade Myofibroblastic Sarcoma Occurred in the Scalp. Journal of Korean Neurosurgical Society, 2015; 58(4): 385-388.
- Zhang Shikun, et al. Low-grade myofibroblastic sarcoma of the orbit: A case report and literature review. Medicine, 2017; 96(51): e9172.
- Jayasooriya Primali Rukmal, et al. Low-Grade Myofibroblastic Sarcoma of the Oral Cavity: A Report of Three Cases Illustrating an Emerging Disease in Children. Dermatopathology, 2021; 8(1): 1-9.
- Tsuyoshi Saito et al. Low-grade myofibroblastic sarcoma of the distal femur. International Journal of Surgery Case Reports, 2013; 4(2): 195-199.
- Peng Long, et al. Low-grade myofibroblastic sarcoma of the pancreas: A case report and literature review. Journal of cancer research and therapeutics, 2018; 14(Supplement): S796-S799.
- Mikami Yurie, et al. Low-grade myofibroblastic sarcoma arising in the tip of the tongue with intravascular invasion: A case report. Oncology letters, 2018; 16(3): 3889-3894.
- Belgium Márquez-Lobo, et al. Low-grade myofibroblastic sarcoma of the larynx: A case report. Spanish Journal of Pathology, 2016; 49(4): 259-262.
- Hadjigeorgiou Georgios F, Samaras Vasilios, Varsos Vasilios. Low-grade myofibroblastic sarcoma of the thoracic spine: report of an extreme rare case. British journal of neurosurgery, 2017; 31(6): 731-733.
- Wang Lu, et al. Low-grade Myofibroblastic sarcoma: clinical and imaging findings. BMC medical imaging, 2019; 19(1): 36.
- Myong Na-Hye and Min Jun-Won. Low-grade myofibroblastic sarcoma arising in fibroadenoma of the breast-A case report. Diagnostic pathology, 2016; 11(1): 33.
- Ni C, Xu YY, Zhou SH, Wang SQ. Differential diagnosis of inflammatory myofibroblastic tumor and low‑grade myofibroblastic sarcoma: Two case reports with a literature review. J Int Med Res, 2011; 39: 311‑320.
- Qiu Jin-Yu, et al. Low-grade myofibroblastic sarcomas of the maxilla. Oncology letters, 2015; 9(2): 619-625.
- Li J, Huang XY, Zhang B. Low-grade myofibroblastic sarcoma of the liver misdiagnosed as cystadenoma: A case report. World J Gastroenterol, 2022; 28(31): 4456-4462. doi: 10.3748/wjg.v28.i31.4456.
- Niu Rong, et al. Low-grade myofibroblastic sarcoma of gastric cardia on 18F-FDG positron emission tomography/computed tomography: An extremely rare case report. Medicine, 2018; 97(4): e9720.
- Taweevisit Mana, Thorner Paul Scott. Distinctive features of low-grade myofibroblastic sarcoma on aspiration cytology: A case report. Cytopathology: official journal of the British Society for Clinical Cytology, 2018; 29(6): 578-581.
- Maruyama Tessho, et al. Indolent growth of low-grade myofibroblastic sarcoma of the cheek mimics benign lesions: A case report and literature review. Oncology letters, 2017; 13(6): 4307-4314.

