Post-Operative Physical Therapy Management of Recurrence Diffuse Pigmented Villonodular Synovitis
Jawza M Alharbi*
Doctor of Physical Therapy, Medical Rehabilitation Department, Qassim University, Saudi Arabia
Received Date: 26/05/2023; Published Date: 27/09/2023
*Corresponding author: Jawza M Alharbi, Doctor of Physical Therapy, Medical Rehabilitation Department, Qassim University, Saudi Arabia
Abstract
Introduction: Pigmented Villonodular Synovitis (PVNS) is a rare, benign disease of the synovial tissue resulting in synovial hyperplasia, pigment deposition (hemosiderin), and swelling inside the affected joints, tendon sheaths, and bursae. In PVNS, there is no conservative management as PVNS continues to grow without surgical intervention. Currently, there is limited research for physical therapy rehabilitation role in post-operative intervention. This case report describes the outpatient physical therapy management of a patient who underwent a synovectomy to remove a recurrence of diffuse PVNS.
Case Description: A 20-year-old male was referred to physical therapy after a synovectomy to remove the recurrent diffuse PVNS on his left knee. The recurrence of symptoms resulted in difficulty performing activities such as: bed mobility, home-related activities, ascending and descending stairs, and walk longer distances.
Intervention: 12 weeks program, including aerobic, eccentric, and proprioception exercises.
Outcome: Post-operative rehabilitation program had a beneficial impact on improving functional mobility (Lower Extremity Functional Scale score improved from 41/80 to 78/80 and 10 Meters Walk Test score improved from 0.83 m/s to 1.1 m/s.). Post-operative recovery proceeded without complications. He was discharged following visit 22.
Keywords: Pigmented villonodular Synovitis; post-operative; Synovectomy; Rehabilitation.
Abbreviations: PVNS: Pigmented villonodular synovitis; ICF: Iternational Classification of Functioning; ROM: Range of Motion; LEFS: Lower Extremity Functional Scale; MDC: Minimal Detectable Changes 10 MWT: 10 Meter Walk Test NPRS: Numeric Pain Rating Scale
Introduction
Pigmented villonodular synovitis (PVNS) is defined as a rare, benign disease of the synovial tissue characterized by synovial hyperplasia, pigment deposition (hemosiderin), and swelling inside the affected joints, tendon sheaths, and bursae. The prevalence of PVNS estimated at 1.8 patients per million [1].
PVNS can be classified by their location and growth patterns as localized or diffuse [2]. The localized form is characterized by discrete nodular or pedunculated lesions with the incidence of 75% of the cases [3]. The diffuse form has grown patterns and affects the entire synovium of large joints.
The most common joint affected is the knee joint (73-84%) followed by the hip (18.14%) [2]. PVNS equally affects both sex [4]. The onset of PVNS can occur at any age range from 2 to 83 years, but generally between the ages of 20 and 50 years old [2].
The etiology of PVNS remains unclear. Some are believed to be caused by chronic inflammation [1], a neoplastic disorder such as a giant cell sarcoma arising near or inside the synovial space, repetitive trauma, or hemorrhages. According to Xiaomei et al reported 42 of 75 cases had a history of joint trauma [5].
Diagnosing PVNS with imaging modalities is often necessary to confirm the diagnosis. MRI is the golden standard in PVNS diagnosing [6][7]. The MRI findings are synovial membrane and soft tissue proliferation from mild proliferation to extensive masses with hemosiderin sedimentation and bone erosion [6]. These changes are the result of pressure atrophy, the actual invasion of bone, or both [6]. A characteristic feature of PVNS confirms a low signal in both sequences but especially on T2, Joint effusion, synovial hyperplasia has a similar density on radiographs, but they can be easily identified by MRI [2].
The most notable symptoms in the localized form are mild pain, swelling, and mechanical symptoms, such as locking, giving way, and catching, with the presence of a palpable mass [8], while in the diffuse form are mild pain, limitations in range of motion, and episodic joint effusions [8].
The gold standard of treatment of PVNS is has been total synovectomy with arthroscopic surgery or open surgery [2].
Patients with knee PVNS had a significantly higher recurrence ratio (42/175, 24.0%) than those with hip PVNS (3/43, 6.98%) [2].
The purpose of this case report is to describe the postoperative physical therapy management of a patient with recurrent PVNS who underwent arthroscopic excision.
Case Presentation
The patient was a 20-year-old male who was referred to physical therapy after a synovectomy in his left knee. The patient lived with his parents. 11 years prior to this episode of care, the patient was diagnosed with diffuse PVNS in his left knee after a repetitive trauma in his left knee when he played on a bicycle. His symptoms included swelling and pain throughout the knee affecting his ability to perform daily activities. He underwent an open synovectomy of his left knee to remove the PVNS.
The patient had recurrent symptoms of pain and swelling 6 years after the initial treatment, but he was able to manage his symptoms conservatively with ice and rest until this episode of care. The patient had noted increased swelling and pain with walking, going up and downstairs, and with prolonged positioning. He would have preferred to be able to walk longer distances, perform home-related activities, ascending and descending stairs, and bed mobility without pain and swelling; however, because of his complaints the patient abstained from these activities. No medical history or family history was found.
According to the patient’s surgeon, the findings from the patient's MRI included: frond-like synovial mass lesion seen at the suprapatellar and infrapatellar region and the posterior knee, it appears of low signal intensity in T1, heterogeneous high signal intensity in T2 and PD with multiple signals. There are suprapatellar and infrapatellar separated large lobulated joint effusion. There is suprapatellar, infrapatellar, posterior knee, and intercondylar mass (Figure 1).
In Figure 2, the patient journey is presented, ordered on a timeline starting from the time of his complaints.

Figure 1: The patient journey is presented on a timeline starting from the time of his complaints.

Figure 2: The WHO framework of the ICF.
Examination
The WHO framework of the ICF was used to structure and organize the patient history and clinical status, as well as to develop the plan of care (Figure 3). The patient was ambulated independently without using any assistive device. A standing postural screen revealed left calf muscle atrophy. The incisions from the first surgery healed well, but the scars remained observable. The current arthroscopic holes were healing normally, with no signs of infection.
The patient’s pain was measured using the Numeric Pain Rating Scale (NPRS). The NPRS has been found to be a valid and reliable measure for chronic and acute pain [9]. Salaffi et al study found the minimal clinically important difference in numerical rating was 1 point or a reduction of 15.0% [10]. Range of motion (ROM) was measured by using Goniometry. Goniometry is a valid, reliable outcome measure for a range of motion [11]. Knee goniometry has high validity and inter-tester reliability [12]. The minimal detectable change (MDC) of range of motion is 3o to 4o [13]. Strength testing was performed by using manual muscle testing. Manual muscle testing is a valid and reliable outcome measure [14]. Assessment of muscle mass was measured by calf circumference. Calf circumference had excellent reliability [15]. It was measured with seated position with the subject's legs dependent over the side of the examination chair, relaxed, hanging at 90o. The circumference of the calf was measured at 15 cm below the medial palpable joint line using a standard tape measure with 1 mm increments. The calf circumference was measured several times until successive measurements produced the same value. Care was taken to ensure that the calf was not compressed by the examination chair or any other way during the measurement process [15]. In addition to the tests performed during the examination that would be used to monitor the patient’s impairment outcomes, the patient was asked to fill out a Lower Extremity Functional Scale (LEFS), that would be used to monitor his self-reported functional progress throughout the intervention. The LEFS is a validated outcomes measure containing 20 questions about a person's ability to perform everyday tasks. Scoring scale 0-80. All 20 items are scored with a maximum score of 4 for each item. The columns on the scale are summed to obtain a final score. The LEFS scores have an excellent test-retest reliability range between 0.85 and 0.99 [16]. In the review of Saurabh et al., an estimate of the MDC was 6 points and the minimal clinically important difference was 9 points in patients with LEFS [17].
10 Meters Walk Test (10MWT) was also used to assess the gait speed. 10MWT has excellent reliability and validity for comfortable and fastest gait speeds [18]. The participant was instructed to walk between two taped lines on the floor and started walking two meters before the first line. Time was measured while the participant walked the 10-meter distance.

Figure 3: The result of LEFS and left calf circumference at initial assessment, at 6th week, and at 12th week. Abbreviations: LEFS = lower extremity musculoskeletal conditions; 10MWT= 10 Meters Walk Test.
Intervention
The intervention began 3 weeks after surgery, with 2 times sessions per week, and continued for 12 weeks. The patient was seen for 22 visits (including the initial evaluation) for 12 weeks. The intervention was focused on addressing those functional deficits and impairments found during the initial evaluation. The intervention addressed pain, ROM, strength, and functional limitations. The specific interventions used are outlined in Table 1.
Phase 1: postoperative phase (week 1-2)
The most important goals in phase 1 are controlling pain, recovery of ROM, and neuromuscular control to prevent postsurgical complications. Cryotherapy is advised as it reduces postsurgical pain [19]. Early passive and active ROM recovery post-surgery reduces pain, induces the homeostasis of cartilage, improves gait pattern, and prevents quadriceps atrophy and arthrofibrosis [20]. Multidirectional patella mobilizations included as a reason of patellar immobility cause decreased ROM and quadriceps inhibition [20]. Patellar mobilizations are performed in the clinic and independently by patients during their home exercise program. Mobilizations are performed in the medial/lateral and superior/inferior directions. Specific exercises included: heel prop. The patient is instructed to apply passive hamstring stretches in the supine position for 15–30 seconds twice a day with 3 repetitions. Range of motion exercise using a stationary bike allows for early controlled knee range of motion. Duration on the bike should follow a gradual progression from 10 to 30 minutes for the first 2–3 weeks. Isometric including multi-angle isometrics 90 - 60 degrees' knee extension and straight leg-raising (SLR) (4 planes) has advantages for faster recovery of knee ROM and stability [21]. Isotonic and proprioception exercises also began in phase 1 without additional weight, including heel slides, mini squads (25° - 30°), and weight shift. Weight-bearing has a positive effect on facilitating the healing and allowing osseous and soft tissue of the knee to respond to normal physiological loading [22]. Shifting body weight was performed in the medial/lateral direction and diagonal patterns. In this phase, intervention incorporates weight-bearing (closed-chain) activities, such as wall slides and step-ups in pain-free ranges (typically 0°- 60°). The patient is instructed to perform 2 sets of 10 repetitions and hold the position for 2 to 3 seconds (Table 1).
Phase 2: intermediate postoperative (weeks 2-3)
Continue to reduce pain, maintain full extension, and flexion within 10 degrees of the contralateral side. Started with 20 minutes of light-intensity aerobic exercise (50–60% of maximal heart rate) using a cycle ergometer. Isometric strength training increasing in intensity. Additional intervention continues with phase I interventions included: calf raises, lumbopelvic strengthening: side-lying hip external rotation-clamshell, plank. By approximately the end of week 3, the patient is instructed to squat approximately 25° to 30° while stabilizing the tilt board to obtain the greatest amount of hamstring and quadriceps co-contraction, performed for 2 sets of 10 repetitions and hold the position for 2 to 3 seconds and progress the repetitions [23] (Table 1).
Phase 3: late post-operative (week 4- 8)
The patient completed three weeks of phase I and phase II exercises that focused on controlling pain, gaining a full range of motion of the knee, and attaining basic quadriceps function (Table 1). Progress the aerobic exercise intensity to (65-70% of maximal heart rate) using a cycle ergometer for 20 minutes with progression to 30 minutes. Beginning in the eccentric exercise (ECC). ECC training stimulates muscle hypertrophy and improves muscle strength for populations ranging from athletes to patients in physical rehabilitation [24]. The patient was instructed to perform the eccentric exercises with his left lower extremity only and to use both lower extremities during the concentric exercises. In all resistance exercises, the patient performed 2 sets 60% of ten repetitions maximum (10RM) for 15 repetitions. As proprioception improves, the single-leg stance is performed on flat ground and progress to unstable surfaces, and lateral lunge drills in a straight plane (Table 1).
Phase 4: transitional (8-12 weeks)
Continue to progress the aerobic exercise intensity to 75% of maximal heart rate using a cycle ergometer for 20 minutes with progression to 30 minutes. Progress eccentric exercises into 2 set 75% of 10RM for 10 repetitions.
Squat to a chair, Lateral lunges were added. Single leg progression into single leg press, slide board lunges: retro and lateral, step-ups and step-ups with march, lateral step-ups, step-downs, single-leg squats, single-leg wall slides. Lateral lunges are progressed to multiple plane/diagonal lunges, lateral lunges with a rotation, and lateral lunges onto foam (Table).
Table 1: The phases of rehabilitation.

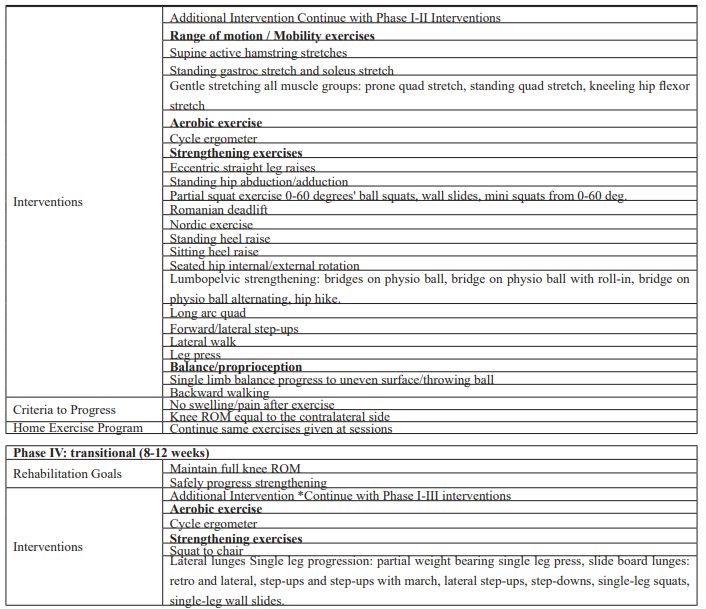
Table 2: The results of left knee ROM and NPRS at initial assessment and at 12th week.

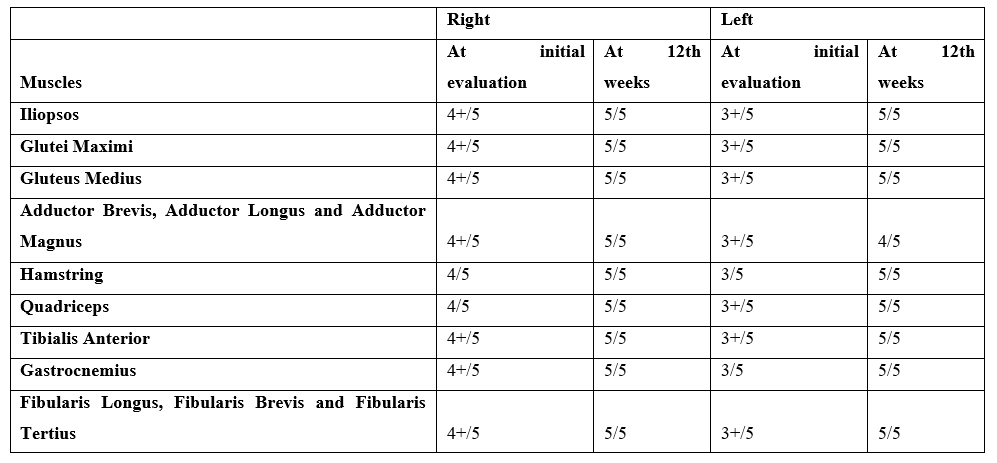
Table 3: The results of MMT.
Outcome
Over the course of the patient’s 12-week rehabilitation program, reported pain on a NPRS, knee ROM measurements, MMT, left calf circumference, and 10MWT: were recorded at initial evaluation and at week 12. In addition, the patient completed the LEFS at the initial evaluation, on week 6 and on week 12. These measurements and scores are shown in Table 2, Table 3, and Figure 4. By the third visit, the patient’s left knee ROM had improved, and by visit 8, the patient was considered to have normal left knee ROM. More importantly, the patient was reporting no pain by visit 6. There may be an association between the patient’s score improvements in LEFS and the fact that he was no longer reporting pain by visit 6, therefore, the patient’s largest score increases across the LEFS were seen in items pertaining to those activities the patient reported as his most painful at initial evaluation. These painful activities included: squatting, ascending/descending stairs, rolling over in bed, lifting an object from the floor, standing for one hour, walking for 1 kilometer and a half, house-related activities, and sports-related activities. The Standard Error of Measure (SEM) for the LEFS +3.9 points [16]. In considering the SEM for this functional scale, it is likely that changes in measure from initial evaluation to week 6 (from 41 to 57), as well as from initial evaluation to week 12 (from 41 to 78) can be considered improvements beyond measurement error (Figure 4). Also, the walking speed showed improvement from the initial evaluation to the 12th week (from 0.83 m/s to 1.1 m/s) by 0.27 m/s as this is greater than 0.13 m/s, which is the MDC for this outcome [25]. Furthermore, the calf circumference was improved from 34 cm to 36.82 cm (Figure 4). The patient has successfully met the rehabilitation goals set out in his initial evaluation and he was discharged following visit 22. He was provided with verbal and written instruction in a home exercise program (HEP) comprised of the same exercise given at sessions.
Discussion
Pigmented Villonodular Synovitis (PVNS) is a rare benign proliferative condition that affects synovial membranes of joints, bursae or tendons caused by neoplastic synovial proliferation with hemosiderin deposition. A retrospective study of seventy-five cases showed that 68 cases had pain, 55 cases had swelling, 61 cases had limited joint range of motion, and there were 42 cases had a history of joint trauma [5]. A review of 20 cases reported that pain was present in 19 of 20 cases, joint swelling was found in 11 cases, and 12 patients complained of repeated joint effusions [26]. The pain and swelling cause moderate to severe range of motion limitations in the affected area [27] [28]. As the disease progress, leading to joint stiffness and joint destruction [26].
The gold standard of treatment of PVNS is has been total synovectomy with arthroscopic surgery or open surgery [29,7]. The surgical intervention goals for PVNS are to restore the function of the joint and to prevent the destruction of articular cartilage by removing the damaged joint lining and the mass. Although, even the surgical intervention was applied there are post-operative complications such as reduced range of motion [30], peripatellar pain, articular effusion, and persistent quadricipital muscle atrophy [31].
The case report illustrates the physiotherapy management followed arthroscopic synovectomy to remove the recurrent PVNS. The result indicates that case report showed marked improvement in range of motion and strength, resulting in improved functional mobility and the ability to perform activities of daily living. Before surgery and physical therapy intervention, the patient had difficulty performing activities of daily living, including home-related activities, bathing, caring object from the floor, ascending and descending stairs, and bed mobility due to pain, chronic swelling.
A limitation of this case report is that there is limited of a standardized physiotherapeutic approach to the treatment of PVNS makes it difficult to recommend particular forms of physiotherapy in particular cases, therefore, a clear need for future randomized control trials in this area.
The evaluation of this patient was completed before physical therapy management of the case. Pain measurement, range of motion, strength, and calf circumference were used during the evaluation. A qualitative outcome measure could have been included to better record the status of the function. Examples of outcome measures applicable to this case include the 10MWT and LEFS.
Conclusion
The findings of this case report suggest that for this individual with PVNS, post-operative interventions focusing on aerobic, eccentric, and proprioception exercises provided relief of the patient’s symptoms.
Declaration
Consent for publication: Written informed consent was obtained from the patient for publication of this case report and any accompany images. A copy of the written consent is available upon request.
Competing interests: No competing interests.
Acknowledgements and funding: The author would like to thank the patient for his participation in this study. The authors received no financial support for this research and/or publication of this case report. Equipment was loaned from the Department of Physical Therapy at Qassim University.
References
- Myers BW, Masi AT, Feigenbaum Pigmented villonodular synovitis and tenosynovitis: A clinical epidemiologic study of 166 cases and literature review. Medicine, 1980; 59: 223-238.
- Plos One: Xie G, Jiang N, Liang C, Zeng J, Chen Z, Xu Q, et al. Pigmented Villonodular Synovitis: A Retrospective Multicenter Study of 237 Cases, 2015.
- Verspoor FG, Geest IC, Vegt E, Veth RP, Graaf WT, Schreuder Pigmented villonodular synovitis: current concepts about diagnosis and management. Future Oncol, 2013; 9: 1515-1531.
- Murphey MD, Rhee JH, Lewis RB, Fanburg-Smith JC, Flemming DJ, Walker EA. Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics, 2008; 28: 1493-1518.
- Ma X, Shi G, Xia C, Liu H, He J, Jin Pigmented villonodular synovitis: Aretrospective study of seventy five cases (eighty one joints). International Orthopaedics, 2013; 37: 1165–1170.
- Cheng XG, You YH, Liu W, Zhao T, Qu MRI features of pigmented villonodular synovitis (PVNS). Clinical rheumatology, 2004; 23: 31-34.
- Mohey N, Hassan Feasibility of MRI in diagnosis and characterization of intra-articular synovial masses and mass-like lesions. Egyptian Journal of Radiology and Nuclear Medicine, 2020; 51: 1-11.
- Kim SJ, Shin SJ, Choi NH, Choo ET. Arthroscopic treatment for localized pigmented villonodular synovitis of the Clinical Orthopaedics and Related Research, 2000; 379: 224-230.
- Jensen MP, McFarland Increasing the reliability and validity of pain intensity measurement in chronic pain patients. International Association for the Study of Pain, 1993; 55: 195-203.
- Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. European Journal of Pain, 2004; 8: 283-291.
- Norkin CC, White Measurement of joint motion: A guide to goniometry, 2016.
- Gogia PP, Braatz JH, Rose SJ, Norton Reliability and validity of goniometric measurements at the knee. American Physical Therapy Association, 1987; 67: 192-195.
- Boone DC, Azen SP Normal range of motion of joints in male Journal of Bone and Joint Surgery, 1979; 61: 756-759.
- Cuthbert SC, Goodheart On the reliability and validity of manual muscle testing: A literature review. Chiropractic & Osteopathy, 2007; 15: 1-23.
- Carmont MR, Silbernagel KG, Mathy A, Mulji Y, Karlsson J, Maffulli Reliability of Achilles tendon resting angle and calf circumference measurement techniques. Foot and Ankle Surgery, 2013; 19: 245-249.
- Binkley JM, Stratford PW, Lott SA, Riddle The Lower Extremity Functional Scale: scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network, 1999; 79: 371-383.
- Mehta SP, Fulton A, Quach C, Thistle M, Toledo C, Evans NA. Measurement properties of the lower extremity functional scale: A systematic Journal of Orthopaedic & Sports Physical Therapy, 2016; 46: 200-216.
- Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and Age and Ageing, 1997; 26: 15-19.
- Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee The American Journal of Sports Medicine, 2001; 29: 288-291.
- DeHaven KE, Cosgarea AJ, Sebastianelli WJ. Arthrofibrosis of the knee following ligament surgery. Instructional Course Lectures, 2003; 52: 369-381.
- Shaw T, Williams MT, Chipchase LS. Do early quadriceps exercises affect the outcome of ACL reconstruction? A randomised controlled Australian Journal of Physiotherapy, 2005; 51: 9-17.
- Grinsven S, Cingel RE, Holla CJ, Loon CJ. Evidence-based rehabilitation following anterior cruciate ligament Knee Surgery, Sports Traumatology, Arthroscopy, 2010; 18: 1128-1144.
- Wilk KE, Escamilla RF, Fleisig GS, Barrentine SW, Andrews JR, Boyd A comparison of tibiofemoral joint forces and electromyographic activity during open and closed kinetic chain exercises. The American Journal of Sports Medicine, 1996; 24: 518-527.
- Isner-Horobeti ME, Dufour SP, Vautravers P, Geny B, Coudeyre E, Richard Eccentric exercise training: modalities, applications and perspectives. Sports medicine, 2013; 43: 483-512.
- Barthuly AM, Bohannon RW, Gorack W. Gait speed is a responsive measure of physical performance for patients undergoing short-term Gait & Posture, 2012; 36: 61-64.
- Houdek MT, Scorianz M, Wyles CC, Trousdale RT, Sim FH, Taunton MJ. Long-term outcome of knee arthroplasty in the setting of pigmented villonodular synovitis. The Knee Journal, 2017; 24: 851-855.
- Brahmi M, Vinceneux A, Cassier PA. Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Current Treatment Options in Oncology, 2016. doi:10.1007/s11864-015-0385-x.
- Al Farii H, Zhou S, Turcotte R. The surgical outcome and recurrence rate of tenosynovial giant cell tumor in the elbow: A literature Journal Shoulder Elbow Surgery, 2019; 28: 1835-1840.
- Cheng YH, Lin YH, Tseng IC, Chan YS. A case series of intra-articular diffuse pigmented villonodular synovitis of the knee: Prognosis of complete synovectomy under arthroscopic Journal of Orthopaedic Surgery, 2021.
- Nakahara H, Matsuda S, Harimaya K, Sakamoto A, Matsumoto Y, Okazaki K, et al. Clinical results of open synovectomy for treatment of diffuse pigmented villonodular synovitis of the knee: Case Series and Review of The Knee Journal, 2012; 19: 684-687.
- De Carvalho LH, Soares LF, Gonçalves MB, Temponi EF, Silva O. Long-term success in the treatment of diffuse pigmented villonodular synovitis of the knee with subtotal synovectomy and The Journal of Arthroscopic & Related Surgery, 2012; 28: 1271-1274.

