Successful Treatment of Apathy with Electroconvulsive Therapy
Gerasimos N Konstantinou1,2, Alisson P. Trevizol1,2,3, Angela Golas1,2,4 and Daniel M Blumberger1,2,3,*
1Department of Psychiatry, University of Toronto, Canada
2Centre for Addiction and Mental Health, Canada
3Temerty Centre for Therapeutic Brain Intervention and Campbell Family Research Institute, Canada
4Geriatric Mental Health Clinic, Centre for Addiction and Mental Health, Canada
Received Date: 22/05/2023; Published Date: 14/09/2023
*Corresponding author: Daniel M. Blumberger, MD, MSc, FRCPC, Medical Head and Director, Temerty Centre for Therapeutic Brain Intervention Centre for Addiction and Mental Health, Professor, Department of Psychiatry, University of Toronto, Mailing: 1025 Queen St. W. Toronto, ON, M6J 1H4, Canada
Abstract
Apathy is a debilitating syndrome associated with many neurological and psychiatric disorders. Although the underlying mechanisms of apathy remain unclear, the impact of apathy on quality of life is substantial. The management of apathy and apathy-like behaviors remains challenging in daily clinical routine. There is a lack of consensus with respect to treatment, with approaches typically focusing on the management of the underlying primary disorder.
We report on a case of a 56-year-old female patient who presented with prominent apathy and treated with a course of right unilateral ultra-brief electroconvulsive therapy (RUL-UB ECT). Although ECT has potential to worsen cognition, in particular short-term memory, which may manifest as a symptom of apathy, in our case the patient exhibited significant improvement in apathy after treatment.
This case highlights the challenges of treating patients presenting with apathy features and that ECT should be considered in complex presentations due to its ability to resolve symptoms that are not addressed by other treatment modalities.
Keywords: Apathy; Electroconvulsive Therapy; Cognitive impairment; Treatment
Introduction
The term apathy derives from the Greek word 'pathos', which means passion. Although the concept of apathy lacks specificity and conceptualizations of apathy vary in the literature, there is general agreement for a triadic substructure, including cognitive/affective, behavioral, and emotional dimensions [1]. The key features of apathy consist of reduced interest and participation in the main activities of daily life, lack of initiative, lack of motivation not attributable to a diminished level of consciousness, cognitive impairment, or emotional distress, a trend towards early withdrawal from initiated activities, indifference, and flattening of affect [1].
Apathy is a debilitating syndrome associated with many neurological and psychiatric disorders, including Alzheimer's disease, Parkinson's disease, vascular dementia, stroke, traumatic brain injury, frontotemporal dementia, major depression, and schizophrenia [1,2]. Apathy is an under-researched and often ignored neuropsychiatric symptom with substantial impact on quality of life, resulting in poor prognosis, accelerated cognitive decline, increased mortality and functional disability [1,3]. There is a lack of consensus regarding the treatment of apathy, with treatment approaches typically focusing on the management of the underlying disorder. The differential diagnosis of the underlying primary disorder is of paramount importance given that pharmacological approaches vary [4,5], and carry the potential to worsen the symptoms of apathy [4]. In this regard, the use of ECT in patients who have a primary neurocognitive disorder can potentially exacerbate facets of cognition particularly short-term memory, which may clinically manifest as a symptom of apathy. In a prospective cohort study, the researchers examined the course of apathy in patients with late‐life depression treated with ECT and the results suggested that apathy was seen in almost two third (58.9%) of all participants after treatment with ECT despite the remission of depressive symptoms [6]. It has also been suggested that various non‐pharmacological interventions and multidisciplinary programs might offer alternative treatment options for patients with apathy, based on a patient-centered approach that considers the patient's preferences, individual needs, environmental factors, targeted lifestyle modifications and context interventions, including exercise, leisure activities, cognitive stimulation, and social activities, with promising results [7].
Herein, we report on the case of a 56-year-old female patient who presented with prominent apathy who was treated with a course of right unilateral ultra-brief electroconvulsive therapy (RUL-UB ECT).
Case Description
Ms. A initially presented at our service with a 3-year history of severe apathy and less prominent depressive symptoms, likely triggered by stressors at work, and refractory to several pharmacotherapy interventions including several selective serotonin reuptake inhibitors (SSRIs), two serotonin and noradrenaline reuptake inhibitors (SNRIs), a tricyclic antidepressant (TCAs), antipsychotic augmentation, and stimulant augmentation. According to family members, her initial presentation was a precipitous (over days) manifestation of apathy, displaying a flat affect and impaired executive function, described as an inability to initiate tasks or make decisions. At baseline, Ms. A. was fastidious with respect to work (in a managerial role), hygiene, and appearance and performed all her activities of daily living (ADLs) and instrumental activities of daily living (IADLs) without difficulty. Six months after the onset of her illness, Ms. A. was admitted for a suicide attempt by drug overdose, returning to her previous state of significant apathy upon discharge.
On interview, Ms. A. unable to define her mood as sad or low, “felt nothing” and appeared perplexed about her feelings. Her chief complaint was “some memory impairment,” but was unable to elaborate further. She denied mood reactivity or diurnal variation. Her sleep had been preserved, and her appetite was low. Ms. A. described significant anticipatory anxiety prior to social interactions. She became increasingly anxious and reluctant to complete ADLs/IADLs, apparently stemming from her concern about executing any task properly, requiring prompting from her husband. Her functional status remained impaired, citing lack of interest in shopping, going to restaurants, or social interaction, spending 10-12 hours per day playing games on her laptop, inconsistent with her baseline level of functioning. Notably, she was able to solve most of the computer game problems, such as chess and solitaire, without difficulty. In terms of ADLs, she required cueing to eat, bathe or shower. Ms A. became dependent upon her husband to select her clothing due to her degree of indecisiveness. Her family also described a dramatic personality change involving a child-like demeanor with an altered tone of voice.
She denied hallucinations in all domains and somatic, persecutory or nihilistic delusions. She denied use of alcohol or other recreational substances.
Ms. A had a normal birth without complications and achieved developmental milestones on time. She gave birth to her two children at age 28 and 30, with normal delivery and no perinatal or postpartum health issues reported. She successfully completed College. She underwent menopause between the ages of 51-55. She denied a past psychiatric history apart from a single panic attack, which was situational and work-stress related, that occurred seven years prior to the onset of apathy symptoms. Family history was significant for the suicide of her father, postpartum depression (mother), and depression and anxiety in a maternal cousin. Concerning her medical history, Ms. A was diagnosed with obstructive sleep apnea in the past no longer needed cPAP due to weight loss. There is no history of any hearing or vision impairment.
Given her history and her clinical presentation, the consulting psychiatrist recommended a neurological examination and neurocognitive assessment. The neurological assessment revealed no abnormalities. On cognitive testing, Ms. A scored 26/30 on the Montreal Cognitive Assessment (MoCA) version 7.1 [8], with deficits in the domains of language (minus two points: sentence repetition, minus one point for phonemic fluency ((generated 6 “F” words)) and orientation (minus one point). She scored 17/18 on the Frontal Assessment Battery (minus one point for lexical fluency), correlating to a negative screen for frontal lobe dementia (cutoff >12). She achieved a total score of 282 on the Toronto Cognitive Assessment – TorCA (normal limits >287; all values normed for ages 50-59) [9], with borderline impaired performance in the domains of memory, delayed recognition [20/21, (normal limits 21)], working memory/executive control [103 (normal limits >105)] and language [75 (normal limits >78)]. Performance in orientation, memory immediate recall, delayed recall, and the visuospatial domains were within normal limits, normed to age. The brain MRI showed non-specific, microangiopathic white matter changes that did not point towards a specific pathology.
Given the onset and course of her illness, the cognitive testing results, the neuroimaging findings, the lack of fluctuations consistent with delirium, and the high index of suspicion that psychiatric symptoms, rather than a major neurocognitive disorder, more likely accounted for her clinical presentation, we offered a course of RUL-UB ECT treatment. Apathy symptoms were assessed with the Apathy Evaluation Scale – AES [10]. Three AES sub-scale scores were collected, with each sub-scale based on the evaluation of a different respective reporter: the subject or self-report (AES-S); her husband (AES-I); and the clinician (AES-C). Cognitive function prior to ECT was assessed with the Montreal Cognitive Assessment - MoCA, version 7.1 [8], mood symptoms with the Patient Health Questionnaire 9 - PHQ-9 [11], and the Quick Inventory of Depressive Symptomatology – QIDS [12], and anxiety symptoms with the Generalized Anxiety Disorder 7-item (GAD-7) Scale [13].
Ms. A received 11 sessions of an acute RUL-UB ECT course (three times per week), followed by a 9-session maintenance RUL-UB ECT course (intervals up to six weeks), at a dose of 230.4 mC after seizure threshold titration, using the following parameters: pulse width, 0.3 msec: frequency, 80 Hz; duration, 6 seconds. Anesthesia was induced with methohexital 70 mg and muscle relaxation achieved with succinylcholine 40 mg and granisetron 1mg was added to suppress the induced nausea. No medication changes occurred during the ECT treatment course. She tolerated treatment well and did not experience adverse effects.
The treatment course led to a notable response in depression and apathy, as indicated by the improvement of all three AES scores (Figure 1). The baseline PHQ-9 score of 15 and the baseline QIDS score of 11 both improved to 5 at the end of the course indicating remission, while GAD-7 baseline scores remained relatively stable, indicating mild anxiety. There was no difference between the baseline and final MoCA scores of 28/30 indicating a normal intact cognitive function, although there was an expected cognitive decline during the acute RUL-UB ECT course (24/30), followed by an improvement during the maintenance course (Figure 2). Subjectively, the patient reported a remarkable improvement in all the domains of her functionality, including ADLs/IADLs, personal hygiene, engaging in conversations with other people, verbal fluency, socializing.
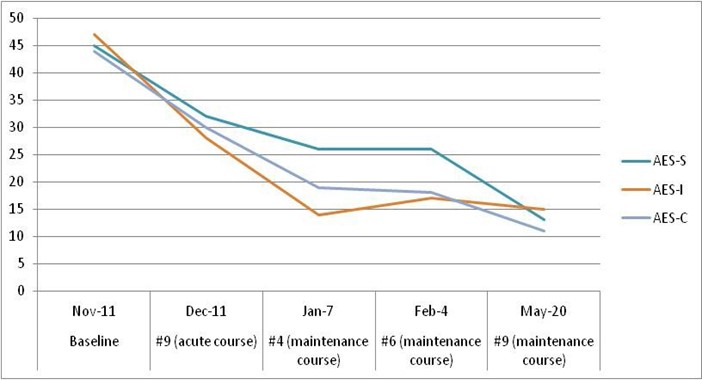
Figure 1: Apathy Evaluation Scale (AES) scores during the treatment course, based on the evaluation of a different respective reporter: the subject or self-report (AES-S); patient’s family member (AES-I); and the clinician (AES-C).
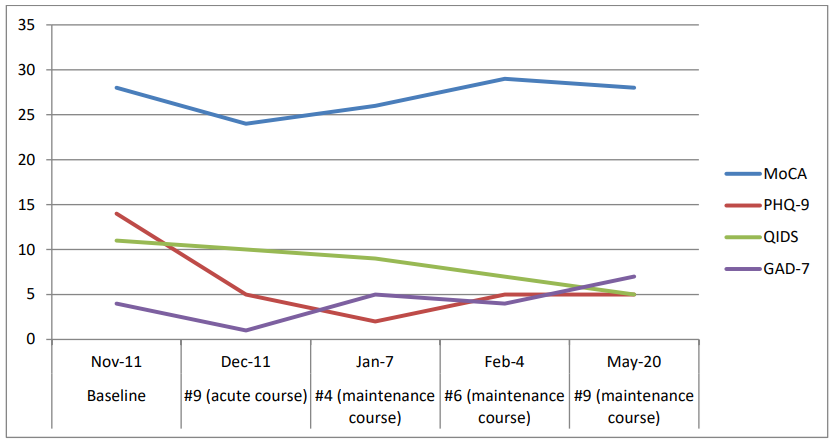
Figure 2: Montreal Cognitive Assessment (MoCA - Version 7.1), Patient Health Questionnaire-9 (PHQ-9) (REF), Quick Inventory of Depressive Symptomatology (QIDS), and Generalized Anxiety Disorder 7-item (GAD-7) Scale scores during the treatment course.
Discussion
To our knowledge, this is the first reported case in literature of apathy treated with a course of RUL-UB ECT course. Dopamine represents a useful and rational target for the treatment of apathy across the wide range of psychiatric and neurological disorders [1]. There is extensive evidence that a dopaminergic deficit, or selective lesions to the mesocorticolimbic system, results in less-motivated behavior, which resembles the behavior of patients with apathy. Focal brain lesions in the frontal lobes, thalamus or basal ganglia can be sufficient to cause apathy, consistent with the role of these brain regions in normal goal-directed behavior and emotional processing [4]. A recent study suggests that apathy is strongly associated with disruption particularly of dorsal anterior cingulate cortex, ventral striatum and connected brain regions in neurodegenerative disorders. Remarkably, these changes were consistent across clinical disorders and imaging modalities [14].
Agents involved in the cholinergic neural pathway, alone or in combination with another pharmacological agent (cholinergic precursor) or glutamatergic agent (memantine), as well as medications having effects on the dopaminergic neural pathway (e.g., methylphenidate and bupropion, dopamine agonists, L-Dopa), seem to be efficient in improving apathy symptomatology, despite conflicting results [4]. Clinically, depression and apathy share several overlapping symptoms such as reduced interest, decision making and initiative that can lead to inaccurate diagnoses, and consequently inappropriate treatment approaches [15]. Antidepressants such as SSRIs and SSNRIs (e.g., duloxetine) used as first-line therapy for depression may worsen apathy symptoms, given their propensity to increase apathy severity [4]. ECT enhances dopamine‐pathways to a greater extent than antidepressants [16], which might explain the notable improvement in the apathy symptomatology.
Conclusion
The management of apathy and apathy-like behaviors remains challenging in clinical practice. This case highlights the challenges when treating patients who present with neuropsychiatric symptoms including depression, apathy and cognitive impairment. In addition, the consideration of ECT in complex presentations of depression with treatment resistance should remain part of the treatment plan due to its ability to resolve symptoms that are not addressed by other treatment modalities.
Funding: This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of Interest: GNK, APT, and AG declare that they have no conflict of interest. DMB has received research support from the CIHR, NIH, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd., and he is the principal site investigator for three sponsor-initiated studies for Brainsway Ltd. He receives in-kind equipment support from Magventure for investigator-initiated research. He received medication supplies for an investigator-initiated trial from Indivior.
References
- Chase TN. “Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment.,” Neurotox. Res., 2011; 19(2): pp. 266–278. doi: 10.1007/s12640-010-9196-9.
- Lanctôt KL, et al., “Apathy associated with neurocognitive disorders: Recent progress and future directions.,” Alzheimers. Dement., 2017; 13(1): pp. 84–100. doi: 10.1016/j.jalz.2016.05.008.
- Starkstein SE, Leentjens AFG. “The nosological position of apathy in clinical practice.,” J. Neurol. Neurosurg. Psychiatry, 2008; 79(10): pp. 1088–1092. doi: 10.1136/jnnp.2007.136895.
- Bogdan A, Manera V, Koenig A, David R. “Pharmacologic Approaches for the Management of Apathy in Neurodegenerative Disorders.,” Frontiers in pharmacology, 2019; 10: p. 1581. doi: 10.3389/fphar.2019.01581.
- Harrison F, Aerts L, Brodaty H. “Apathy in Dementia: Systematic Review of Recent Evidence on Pharmacological Treatments.,” Curr. Psychiatry Rep., 2016; 18(11): p. 103. doi: 10.1007/s11920-016-0737-7.
- Carlier A, et al., “The course of apathy in late-life depression treated with electroconvulsive therapy; a prospective cohort study.,” Int. J. Geriatr. Psychiatry, 2018. doi: 10.1002/gps.4917.
- Theleritis C, Siarkos K, Politis AA, Katirtzoglou E, Politis A. “A systematic review of non-pharmacological treatments for apathy in dementia.,” Int. J. Geriatr. Psychiatry, 2018; 33(2): pp. e177–e192. doi: 10.1002/gps.4783.
- Nasreddine ZS, et al., “The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment.,” J. Am. Geriatr. Soc., 2005; 53(4): pp. 695–699. doi: 10.1111/j.1532-5415.2005.53221.x.
- Freedman M, et al., “The Toronto Cognitive Assessment (TorCA): normative data and validation to detect amnestic mild cognitive impairment.,” Alzheimers. Res. Ther., 2018; 10(1): p. 65. doi: 10.1186/s13195-018-0382-y.
- Marin RS, Biedrzycki RC, Firinciogullari S. “Reliability and validity of the Apathy Evaluation Scale.,” Psychiatry Res., 1991; 38(2): pp. 143–162. doi: 10.1016/0165-1781(91)90040-v.
- Kroenke K, Spitzer RL, Williams JB. “The PHQ-9: validity of a brief depression severity measure.,” J. Gen. Intern. Med., 2001; 16(9): pp. 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x.
- Rush AJ, et al. “The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression.,” Biol. Psychiatry, 2003; 54(5): pp. 573–583. doi: 10.1016/s0006-3223(02)01866-8.
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. “A brief measure for assessing generalized anxiety disorder: the GAD-7.,” Arch. Intern. Med., 2006; 166(10): pp. 1092–1097. doi: 10.1001/archinte.166.10.1092.
- Le Heron C, Apps MAJ, Husain M. “The anatomy of apathy: A neurocognitive framework for amotivated behaviour.,” Neuropsychologia, 2018; 118(Pt B): pp. 54–67. doi: 10.1016/j.neuropsychologia.2017.07.003.
- Iacobacci C. “Common and Different Features Between Depression and Apathy in Neurocognitive Disorders,” Clin. Exp. Psychol., 2017; 03(03): p. 163. doi: 10.4172/2471-2701.1000163.
- Nutt DJ. “The role of dopamine and norepinephrine in depression and antidepressant treatment.,” J. Clin. Psychiatry, 2006; 67(Suppl 6): pp. 3–8.

