Role of Laboratory in the Management of HBV Reactivation in Rheumatic Diseases
Margarita Prifti-Kurti1,2,*
¹Departament of Laboratory, Medical University of Tirana, Albania
²Diagnostic Center Optimus, Tirana, Albania
Received Date: 17/05/2023; Published Date: 07/09/2023
*Corresponding author: Margarita Prifti-Kurti, Departament of Laboratory, Medical University of Tirana, Diagnostic Center Optimus, Tirana, Albania
Abstract
In patients with rheumatic diseases undergoing immunosuppressive treatment, hepatitis B virus reactivation (HBVr) has been long recognized as a major treatment-related adverse event with substantial morbidity and mortality. HBVr is easily preventable with appropriate screening and monitoring strategies. A forty three-year old female is diagnosed on 03.03.2022 with diffuse scleroderma. She started treatment with Methotrexate (25 mg/week) and Prednisone (40 mg/day) for 6 months. No screening for HBV was performed before starting treatment. After 7 months was performed the first screening which included ALT and AST. The enzymes resulted higher than normal range. Then the other examinations were requested to diagnose or exlude autoimmune hepatitis, HCV, HBV. All results confirmed HBVr, second stage. Immunosuppressive treatment was discontinued and Antiviral treatment started on 28.10.2022. Three months later the HBsAg was 5 times less than the previous measurement. Four months later the transaminases were normalized. We can say that HBVr is a potentially complication that depend on baseline HBV status of the patient and on the therapeutic agent used. So before immunosuppressive treatment, serological tests should be performed to assess the status of HBV infection and monitoring during therapy must be performed with serial measurements of ALT, HBsAg, DNA-HBV (every 3 - 6 months).
Keywords: Hepatitis B Virus reactivation; American Gastroenterological Association; Antimitochondrial; Smooth muscle antibody
Introduction
Hepatitis B virus (HBV) infection is a major global health problem. This infection continues to be a problem of morbidity and mortality despite the availability of an efficacious vaccine and antiviral treatment [1]. It can cause liver diseases ranging from acute hepatitis to chronic hepatitis, cirrhosis and hepatocellular carcinoma [2,3]. The virus itself does not have a direct cytopathic effect. On the contrary, hepatocellular injury is mediated by innate and adaptive immunity. A strong immune response is associated with viral clearance in acute HBV infection, but is also responsible for hepatocyte damage and fibrosis in the immune active phases of chronic HBV infection [4].
The majority of infected people are unaware that they have chronic HBV infection, or have been exposed to HBV, but is fact that HBV remains present in individuals who have passed the infection. HBV reactivation (HBVr) is a well-recognized complication of immunosuppressive treatment in cancer, rheumatic diseases, and organ transplantation [5]. HBVr is a clinical syndrome characterized by:
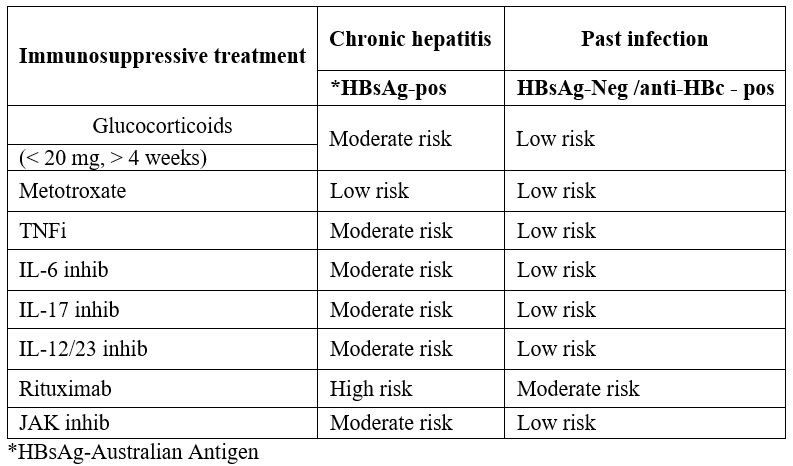
A sudden increase in HBV DNA replication and a moderate increase in ALT 2 - 3 times up to normal range. Reactivation occurs in individuals who have passed HBV (HBsAg-negative) and antibodies (anti-HBc-positive) and in individuals suffering from chronic hepatitis (HBsAg-positive and anti-HBc-positive) [6]. It is generally considered a failure of the immune system's control over HBV replication.This can occur either spontaneously or whenever the immune system is compromised. Factors that influence the risk of HBVr are related to the patient, the virus, and the type and duration of immunosuppression used [7] (Table 1). Recent years, there has been an effort to stratify HBVr risk according to the patient’s serological status and the type and duration of the immunosuppressive treatment used. The American Gastroenterological Association (AGA) classified HBVr risk as low, moderate, and high based on the above factors [8] (Table 2).
Table 1: Factors that influence the risk of HBVr.

Table 2: Type of mmunosuppression and HBVr risk [8-14].
The best way to assess risk of HBVr is serological testing for HBV as follow:
HBsAg, HBeAg, HBV-DNA, anti-HBc total =Positive, anti-HBc IgM. When anti-HBc IgM is negative indicates the HBVr. The anti-HBc IgM positive indicates the acute infection of HBV.
A past HBV infection is assessed by the titer of anti-HBs antibodies.The presence of high-titer anti-HBs significantly reduces the risk of HBVr in immunosuppressive treatments [15].
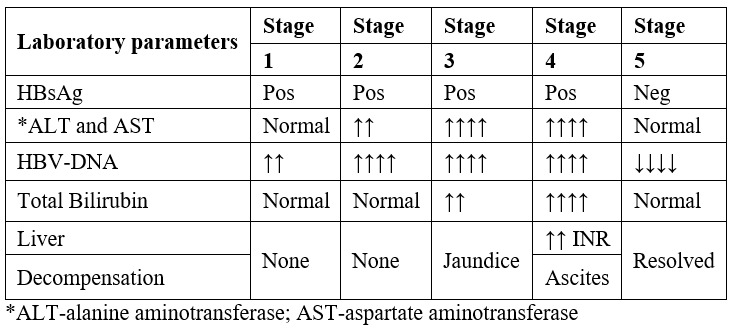
Table 3: Laboratory alterations in HBVr after immunosuppressive treatment [16-18].
Case Report
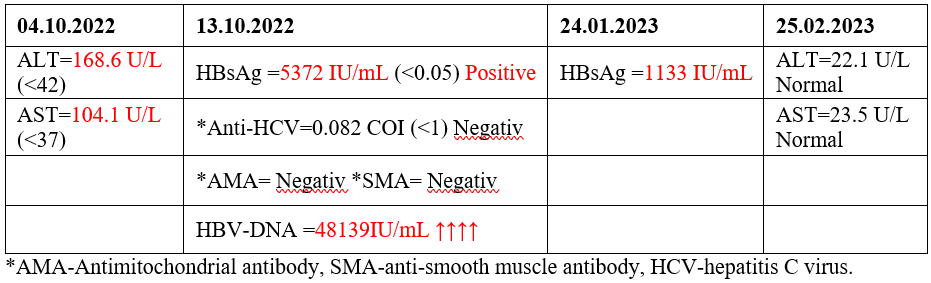
A forty-three-year-old female is diagnosed on 03.03.2022 with diffuse scleroderma. She started treatment with Methotrexate (25 mg/week) and Prednisone (40 mg/day) for 6 months. No screening for HBV was performed before starting treatment. After 7 months was performed the first screening which included ALT and AST. The enzymes resulted higher than normal range (Table 4). Then the other examinations were requested to diagnose or exlude autoimmune hepatitis, HCV, HBV (Table 4). All results confirmed HBVr, second stage (Table 3).
Immunosuppressive treatment was discontinued and Antiviral treatment started on 28.10.2022. Three months later the HBsAg was 5 times less than the previous measurement. Four months later the transaminases were normalized (Table 4).
Table 4. Laboratory tests results.
Management of HBVr for therapy decision
The management of HBVr should be based on individual HBVr risk according to the patient’s HBV status (chronic or resolved infection), but also on the HBVr potential of the immunosuppressive treatment used.
1. HBsAg positive and Anti-HBc positive patients. HBsAg positive patients are at high risk for HBVr from immunosuppressants. In this case, antiviral therapy is recommended before starting immunosuppressants [19-21].
2. HbsAg negative and Anti-HBc positive patients. The dose of immunosuppressants depends on the level of anti-HBs antibodies. High dose corticosteroids require regular monitoring of liver function and viral load as they pose a high risk for HBVr. If an increase in the viral load is observed, antiviral therapy is recommended before treatment with immunosuppressants [19-21].
3.HBsAg negative / Anti-HBc negative / Anti-HBs negative patients (Individuals who have not been exposed to HBV) Hepatitis B vaccine is recommended (individuals who are at risk of being infected with HBV) [19-21].
Conclusion
In patients with rheumatic disease under immunosuppressive treatment and HBV infection, HBVr is a complication that rheumatologists need to be aware of HBVr rates depend on baseline HBV status of the patient and on the therapeutic agent used. So before immunosuppressive treatment, serological tests should be performed to assess the status of HBV infection and monitoring during therapy must be performed with serial measurements of ALT, HBsAg, DNA-HBV (every 3 - 6 months).
References
- Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet,2015; 386: 1546–1555.
- Lazarus JV, Picchio C, Dillon JF, Rockstroh JK, Weis N, Buti M. Too many people with viral hepatitis are diagnosed late - with dire consequences. Nat Rev Gastroenterol Hepatol,2019; 16: 451-452.
- Koutsianas C, Thomas K, Vassilopoulos D. Prevention of HBV reactivation in patients treated with biologic agents. Expert Rev Clin Pharmacol,2016; 2433: 1–11.
- Seto WK, Lo YR, Pawlotsky JM, et al. Chronic hepatitis B virus infection. Lancet,2018; 392: 2313–2324.
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology,2018; 67: 1560–1599.
- Koutsianas C, Thomas K, Vassilopoulos D. Hepatitis B reactivation in rheumatic diseases: screening and prevention. Rheum Dis Clin North Am,2017; 43: 133–149.
- Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology,2017; 152: 1297–1309.
- Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis b virus reactivation during immunosuppressive drug therapy. Gastroenterology,2015; 148: 221–244.e3.
- Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology,2017; 152: 1297–1309.
- Tamori A, Koike T, Goto H, et al. Prospective study of reactivation of hepatitis B virus in patients with rheumatoid arthritis who received immunosuppressive therapy: evaluation of both HBsAg-positive and HBsAg-negative cohorts, J Gastroenterol, 2011; 46: 556–564.
- Tien Y-C, Hsue Y-T, Hung M-H, et al. Changes in hepatitis B virus surface antibody titer and risk of hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients undergoing biologic therapy for rheumatic diseases: a prospective cohort study. Arthritis Res Ther,2018; 20: 246.
- Fukuda W, Hanyu T, Katayama M, et al. Incidence of hepatitis B virus reactivation in patients with resolved infection on immunosuppressive therapy for rheumatic disease: a multicentre, prospective, observational study in Japan. Ann Rheum Dis,2017; 76: 1051–1056.
- Lin Y-C, Lee S-W, Yeh H-Z, et al. The prevalence and risk factors of hepatitis B flares in chronic hepatitis B patients receiving glucocorticoid pulse therapy. Int J Clin Pharm,2018; 40: 169–174.
- Moghoofei M, Mostafaei S, Ashraf-Ganjouei A, et al. HBV reactivation in rheumatic diseases patients under therapy: a meta-analysis. Microb Pathog,2018; 114: 436–443.
- Paul S, Dickstein A, Saxena A, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: a meta-analysis. Hepatology,2017; 66: 379–388.
- Xuan D, Yu Y, Shao L, et al. Hepatitis reactivation in patients with rheumatic diseases after immunosuppressive therapy - A report of long-term follow-up of serial cases and literature review. Clin Rheumatol, 2014; 33: 577–586.
- Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol,2017; 67: 370–398.
- Wong GL-H, Wong VW-S, Yuen BW-Y, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol, 2019.
- Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of a Hepatitis B Vaccine with a Novel Adjuvant. MMWR,2018; 67: 455–458.
- Schwaneck EC, Krone M, Kreissl-Kemmer S, et al. Management of anti-HBc-positive patients with rheumatic diseases treated with disease-modifying antirheumatic drugs-a single-center analysis of 2054 patients. Clin Rheumatol,2018; 37: 2963–2970.
- Hwang JP, Lok AS-F. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol,2014; 11: 209–219.

