Heart Failure (HF) Nurses and Allied Professionals Specialists Contribute to Differential Diagnosis of Patients with HF and Comorbidities
Katerina Philippou1,*, Angela Malaktou2, Martha Kyriakou3, Niki Vouri4, Sotiris Avgousti5, Vasilis Barberis6 and Ekaterini Lambrinou7
1Special Teaching Staff, Department of Nursing, School of Health Sciences, Cyprus University of Technology, Cyprus
2Accident and Emergency Department, Larnaca General Hospital, Larnaca, Cyprus
3Intensive Care Unit, General Hospital of Nicosia, Nicosia, Cyprus
4Accident and Emergency Department, Ammochostos General Hospital, Famagusta, Cyprus
5Assistant Professor, School of Health Sciences, Cyprus University of Technology, Cyprus
6Stasandrou 10, Nicosia, Cyprus
7Professor, School of Health Sciences, Cyprus University of Technology, Cyprus
Received Date: 06/05/2023; Published Date: 24/08/2023
*Corresponding author: Katerina Philippou, RN, BSc (Hons), MSc, PhDc, Special Teaching Staff, Department of Nursing, School of Health Sciences, Cyprus University of Technology, 15, Vragadinou Str, 3041 Limassol, Cyprus
Abstract
Patient presentation: A 76 years old male patient with Heart Failure (HF) enrolled in the nurse-led management program ‘Support Heart’ is presented in the current clinical case. Even though he was well educated and supported through the program, he had several deterioration and re-hospitalizations, during a few months period. The symptoms remained after his discharge and specialist nurses of the program advised him to visit a cardiologist specialist on HF and other specialists as well who diagnosed amyloidosis.
Initial work-up: The patient was introduced to the management program ‘Support Heart’ after he was diagnosed with HF with preserved Ejection Fraction (HFpEF). ‘Support Heart’ program includes specialist nurses and physiotherapists on HF who collaborate with cardiologists; and provide monthly follow-up meetings in which the patients are educated, ask questions, do exercise, walking etc. During the pandemic and the lockdowns (a few months period), the patient was admitted three times within the cardiology ward with dyspnea NYHA III and swelled legs with ulcers. The first meeting of the program after the lockdown was only a few days after discharge. HF nurses during his assessment found that the symptoms were not better and undertook a more detailed medical history when they found out that his brother died from a liver disease. The family history with the unknown disease and the continuing symptoms were the reasons that nurses referred the patient to a HF cardiologist specialist. The cardiologist suspected amyloidosis and asked for further evaluation tests.
Diagnosis and management: The scintigraphy and hematological tests suggested transthyretin amyloidosis. His therapy then was optimized and upgraded with disease specific treatment (tafamidis) for amyloidosis and the clinical presentation of the patient was improved. His NYHA stage became II and the ulcers were much better. The nurses of the ‘Support Heart’ program were informed by the cardiologist about the new therapy and the necessary follow-up treatment.
Follow-up: Amyloidosis is an increasingly recognized but too often underestimated cause of HF. It is often underdiagnosed due to the lack of clinical manifestations. The new possibilities of imaging and tests along with a careful clinical assessment and medical history provide the opportunity for early diagnosis, optimization of the therapy and improved clinical outcomes. The HF nurses of the ‘Support Heart’ program explained to the patient about cardiac amyloidosis and how it changes his treatment and follow-up of the multi-disciplinary team. Then, they made together with the physiotherapist of the program a new program of physical activity since his physical condition along with his mental condition were improved.
Conclusion-Learning points: Specialist nurses and physiotherapists in HF and supportive nurse-led management programs may contribute to differential diagnosis of patients with HF and comorbidities and improve the outcomes of the patient and the coordination of health professional specialists. Patients with HF who are mostly older people with comorbidities need continuing support, evaluation and optimization of therapy.
Keywords: ATTR Amyloidisis; Transthyretin (TTR) Cardiac Amyloidisis; Tafamidis
Introduction
Amyloidosis is a heterogeneous family of diseases induced by deposition of misfolded proteins in the form of amyloid fibrils within the extracellular space of various organs. Amyloid deposits are histologically identifiable by characteristic apple-green birefringence when stained with Congo-Red dye and examined under cross-polarized light.
As confirmation of the amyloid fibril type is essential to direct clinical management and disease-modifying therapy, in such inconclusive cases, the use of immunogold electron microscopy and mass spectrometry confer the greatest sensitivity and specificity for amyloid typing [1,2]. Major advances in imaging such as scintigraphy with bone tracer and cardiac magnetic resonance (CMR) have heralded a non-invasive approach to diagnosis of ATTR-CA, which now may be achieved without recourse to histological demonstration of amyloid in ≈70% of the cases [3].
Transthyretin (TTR) amyloidosis (ATTR amyloidosis), is an underdiagnosed, life-threatening disease characterized by progressive deposition of misfolded or cleaved TTR protein in organs [1,4]. Disease occurs when aggregation of amyloid fibrils in the extracellular space disrupts the structure, integrity and function of the affected tissue. In clinical practice, ATTRwt amyloidosis manifests as a predominant cardiomyopathy [transthyretin cardiac amyloidosis (ATTR-CA)], while ATTRv amyloidosis is typically associated with Polyneuropathy (ATTR-PN) as well as cardiomyopathy [5].
Amyloid deposition in the heart leads to expansion of the extracellular space with associated disruption in myocardial architecture, systolic and diastolic function [6]. The increase in myocardial mass determines a progressively smaller ventricular cavity size, resulting in fixed end-diastolic volume. ATTR-CA is slowly progressive and clinically well tolerated until marked ventricular wall thickening, severe diastolic dysfunction and conduction system disease have occurred [1,4].
Case Report
A 76 years old male patient with HF who was enrolled to the nurse led management program ‘Support Heart’ with reduced ejection fraction admitted for third time in the cardiology ward with dyspnea NYHA III and swelled legs with ulcer. The patient was admitted another two times with deterioration of symptoms during the pandemic and lockdown. He was educated about the HF and was supported for self-management using several educational methods during monthly meetings and telephone follow-up. After the therapy with diuretics and bronchodilators, the patient was discharged, but the symptoms did not improve. It was considered as fatigue due to the existing disease. Moreover, the patient complained that he could not use the walking aid because his wrists were aching. His arterial Blood Pressure (BP) was also lower than usual.
He had a normal BMI but he can’t walk without walking aid. At the first meeting of the program after the lockdown, he was only a few days after the last discharge. HF nurses during his assessment found that the symptoms remained. His BP remained lower than usual and his medication had to be modified. undertook a more detailed medical history when they found out that his brother died from a liver disease. The family history with the unknown disease and the continuing symptoms were the reasons that nurses referred the patient to a HF cardiologist specialist. The cardiologist suspected amyloidosis and asked for further evaluation tests.
Individual history includes coronary disease-multiple PCI, chronic atrial fibrillation, hypertension, and diabetes mellitus. Medication at the period of readmissions included: carvedilol, ezetimibe, metformin, rivaroxaban, amlodipine/valsartan, rosuvastatin, allopurinol, alfuzocin. FBC and Biochemical tests were normal. ECG showed sinus rhythm with first-degree atrioventricular block (Figure 1).
ECHO showed preserved ejection fraction 55%, normal dimension with severe degree of centralized left ventricular wall hypertrophy (LVEDD=42mm, IVS=16mm PW=16mm) (Figure 2).
An osteomedelic biopsy was performed to rule systematic amyloidosis out. It was negative. The patient was referred for heart scintigraphy. Results showed moderately increased myocardial uptake of radiopharmaceuticals (Grade =2) and compatible finding with ATTR amyloidosis (Figure 3).
The therapy then was optimized and upgraded with disease specific treatment (tafamidis) for amyloidosis. Due to the severity and the long term of the disease the improvement was slow. One year later the patient was able to walk without walking aid, his HF symptoms were in remission and he had a marked improvement on his health related -quality of life.
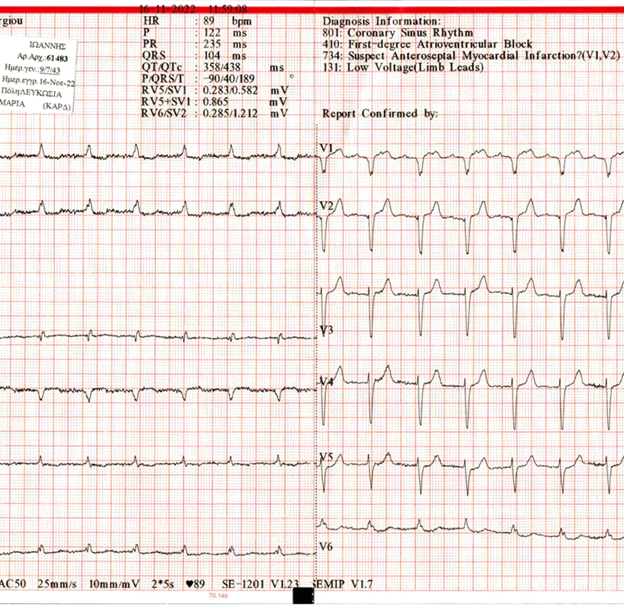
Figure 1
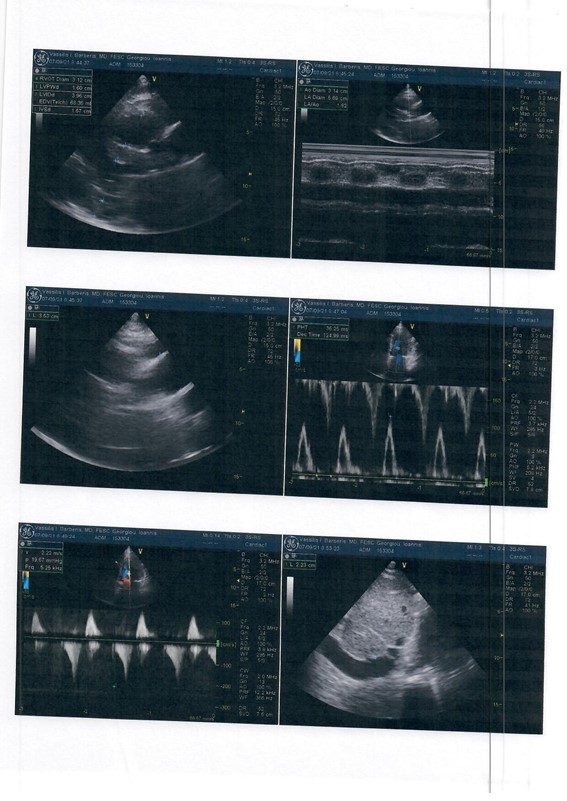
Figure 2
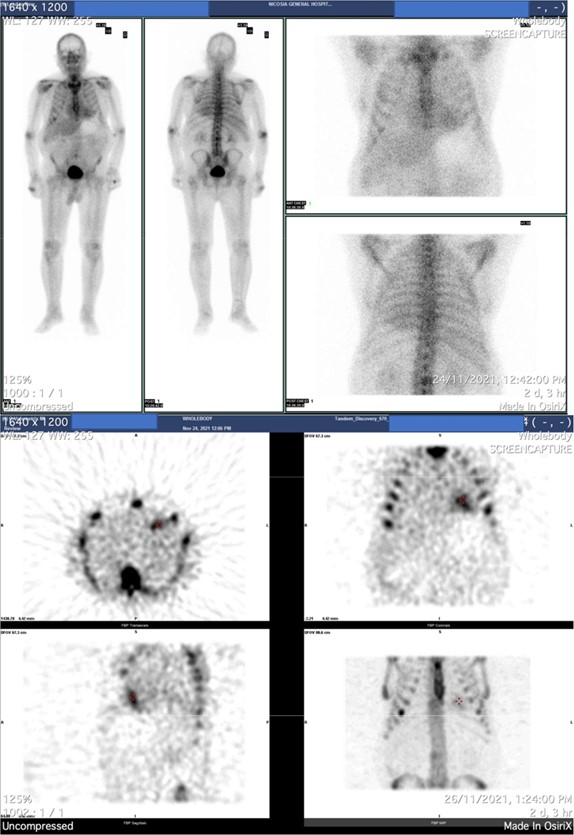
Figure 3
Discussion
Cardiac amyloidosis is frequently misdiagnosed, so the patients in many cases don’t have the opportunity for appropriate optimization of the treatment management of the disease [6]. The relevance of the present clinical case was based on the ESC Clinical Guidelines for the diagnosis of Cardiac Amyloidosis [4]. The patient of the clinical case had left ventricular wall thickness ≥ 12mm (=16 mm) from the echocardiogram and four “red flugs” that were the following: 1. Diagnosed patient with Heart Failure (HFpEF = 55%) older than 65 years old, 2. Hypotensive patient and previously was hypertensive 3. Ppatient with bilateral carpal tunnel syndrome and 4. Possible family history.
Early diagnosis and clinical suspicion
Early diagnosis of amyloidosis by using non-invasive testing (first and foremost scintiscan with bone markers) should always be guided by clinical suspicion but should also be supported by a multidisciplinary approach with the aim to improve the prognosis of the condition [6]. In the present clinical case, the patient was involved in a nurse-led supporting care program for the management of HF in Cyprus named “Support Heart”. During the monthly meetings, the nurses of the program observed that during patients’ assessment the symptoms remained after the treatment he received during and after the three times of his hospitalizations. Nurses undertook again a detailed medical and family history. The patient referred that his brother died from an unspecified liver disease and himself had bilateral carpal tunnel syndrome the last months. The carpal tunnel syndrome earns special attention and especially if appears bilateral. In male patients this is highly suggestive of ATTR (up to 50%) and in the studies, it seems to lead up to the cardiac involvement of 5– 7years [6,7]. The nurses of the “Support Heart” team referred the patient to a HF cardiologist specialist who also suspected amyloidosis and asked for further evaluation tests. Therefore, the proper assessment and evaluation by the nurses of the “Support Heart” program leaded to the early and proper diagnosis for cardiac amyloidosis. The role of the multidisciplinary team is fundamental for the early diagnosis of cardiac amyloidosis.
The collaboration of HF nurses and cardiologists’ specialists, is essential and is important that nurses must also be aware and have the clinical suspicion of the symptoms of cardiac amyloidosis. The aim is to contribute to early diagnosis and treatment, since patients with early diagnosis and care, have better outcomes and health related quality of life [8,9].
Conclusion
Cardiac amyloidosis is a condition that is fatal and progressive. [9]. A late diagnosis significantly affects the prognosis of the patients and the possibility of undertaking the proper therapies, so they may lack the possibility to heal or slowing the progression of the disease, despite the fact that new medications are used.
Several “red flags” have been identified and may raise suspicion for the presence of the disease [3,10]. Therefore, if there is the appropriate knowledge and assessment by members of the multidisciplinary heath care team, such as HF nurses, the identification of the complex picture of signs and symptoms related to amyloidosis, makes the early diagnosis appropriate therapeutic procedure possible leading to improved outcomes.
References
- Martinez-Naharro AJ, Baksi PN, Hawkins, Fontana M. “Diagnostic imaging of cardiac amyloidosis,” Nature Reviews Cardiology, 2020; 17(7): pp. 413–426. doi: 10.1038/s41569-020-0334-7.
- Lavatelli F, Vrana JA. “Proteomic typing of amyloid deposits in systemic amyloidosis. 2011; 18(4): pp. 177–182. doi: 10.3109/13506129.2011.630762.
- Gillmore JD, et al., “Nonbiopsy diagnosis of cardiac transthyretin amyloidosis,” Circulation, 2016; 133(24): pp. 2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612/-/DC1.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42(36): 3599-3726.
- Wechalekar D, Gillmore JD, Hawkins PN. “Systemic amyloidosis,” The Lancet, 2016; 387(10038): pp. 2641–2654. doi: 10.1016/S0140-6736(15)01274-X.
- Maurer MS, et al. “Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey),” J Am Coll Cardiol, 2016; 68(2): pp. 161–172. doi: 10.1016/J.JACC.2016.03.596
- Porcari, et al. “Incidence and Characterization of Concealed Cardiac Amyloidosis Among Unselected Elderly Patients Undergoing Post-mortem Examination,” Front Cardiovasc Med, 2021; 8: p. 1680. doi: 10.3389/FCVM.2021.749523.
- Falk RH. “Diagnosis and Management of the Cardiac Amyloidoses,” Circulation, 2005; 112(13): pp. 2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187.
- Kendall H. “Cardiac Amyloidosis,” Crit Care Nurse, 2010; 30(2): pp. 16–23. doi: 10.4037/CCN2009202.
- Sabbour H, et al. “From Clinical Clues to Final Diagnosis: The Return of Detective Work to Clinical Medicine in Cardiac Amyloidosis,” Front Cardiovasc Med, 2021; 8: p. 546. doi: 10.3389/FCVM.2021.644508.
- Witteles RM, et al., “Screening for Transthyretin Amyloid Cardiomyopathy in Everyday Practice,” JACC Heart Fail, 2019; 7(8): pp. 709–716. doi: 10.1016/J.JCHF.2019.04.010.

