Community-Acquired Carbapenem-Resistant Enterobacter Cloacae Cellulitis
Ayesha Shahid1,2,3,* and Gulzar Ahmed4,5,6
1MBBS, Jinnah Sindh Medical University, Pakistan
2House officer, Jinnah Postgraduate Medical Centre, Pakistan
3Medical Officer, Creek General Hospital, Pakistan
4MBBS, Dow University of Health Sciences, Pakistan
5House Officer, Civil Hospital Karachi, Pakistan
6Medical Officer, Indus Hospital, Pakistan
Received Date: 04/05/2023; Published Date: 18/08/2023
*Corresponding author: Ayesha Shahid, MBBS, Jinnah Sindh Medical University, House officer, Jinnah Postgraduate Medical Centre, Medical Officer, Creek General Hospital, Pakistan
Abstract
The purpose of this case report is to describe the presentation of community-acquired, Carbapenem-Resistant Enterobacter Cloacae (CREC) cellulitis in a 35-year-old male. The patient presented with a two-day history of swelling and pain in the right foot associated with fever. The swelling was non-traumatic and rapidly progressive. A diagnosis of cellulitis was produced. The patient was kept on broad-spectrum IV antibiotics (Co-amoxiclav and amikacin) and underwent debridement of the wound. Upon isolation of CREC on tissue culture, fosfomycin was started. Treatment continued after hospitalization, and the patient was kept on outpatient follow-up post-hospitalization. This case study demonstrates one of the first known cases of community-acquired CREC infection. It establishes the rapidly emerging drug resistance in Enterobacter Cloacae species and a singular case of successful treatment, proving that timely recognition, investigation and treatment can lead to a successful outcome with these highly resistant infectious species.
Keywords: Cellulitis; Community-acquired CREC; Carbapenem-resistance; Enterobacter Cloacae; Infectious; Drug resistance; MDR
Introduction
Enterobacter Cloacae (E. Cloacae), is an anaerobic gram-negative bacterium, generally found in the gut and widely found in environments like soil, water and sewage [1]. It is considered to be a common opportunistic infection and is one of the top three causes of hospital-acquired infections in recent years. Enterobacter Cloacae is part of the Enterobacter Cloacae Complex (ECC), which further comprises of Enterobacter hormaechei, Enterobacter asburiae, Enterobacter kobei, Enterobacter ludwigii, Enterobacter nimipressuralis, Enterobacter Mori, etc., which are all widely found in the environment [2].
ECC organisms have been implicated as causative agents in multiple infections including respiratory tract infections, urinary tract infections, hospital-acquired infections, sepsis and wound infections [3]. ECC organisms are intrinsically resistant to penicillins and first and second-generation cephalosporins [4]. However, the recent development of carbapenem resistance among Enterobacteriaceae is an escalating problem of great clinical concern [5].
Colistin is another antibiotic that previously fell out of clinical use but has recently been reapplied for clinical use due to its positive antibacterial activity against MDR (multi drug-resistant) gram-negative bacteria. It is generally considered to be the last line of treatment for severe infection caused by MDR gram-negative bacteria.
This emergence of carbapenem and colistin-resistant strains of ECC poses a huge threat and challenge in terms of infection control. The emergence of carbapenem resistance further increases the risk of the spread and dissemination of such strains.
In recent years there has been an increasing trend in the emergence of carbapenem-resistant ECC strains that have been found in multiple regions around the world [6-8]. This widespread emergence further makes it more difficult to treat MDR strains of CREC (Carbapenem-resistant Enterobacter Cloacae) infections. The added ability of some of these strains to horizontally spread their drug-resistance genes poses an even greater threat to public health.
Case Report
History and examination
An otherwise healthy 35-year-old male presented to the emergency room (ER) of a tertiary care hospital in Badin, Pakistan with the chief complaint of progressive pain and swelling of the right foot for three days. This was associated with intermittent fever for the same duration. The patient had no history of trauma or any penetrating injury. The wound was as seen in Figure 1.
The physical examination findings were as follows, blood pressure 120/90, respiratory rate 18, heart rate 89, temperature 36.2°C. On examination of the foot, generalized swelling of the right foot was seen, with a bullous swelling on the medial aspect of the right foot. No open wounds, insect bites or pus discharge was observed. The movements of the patient were restricted at the foot due to pain, however sensory perception and distal pulses were intact. During the examination of the wound in ER, the blister burst with a discharge of watery fluid as seen in Figure 2.
The patient had no known comorbidities. He also denied any history of allergies, past medical conditions, recent hospitalizations, and surgeries.
Investigation
Baseline investigations revealed values as shown in Table 1, indicating severe infection. Urea and Creatinine were within normal limits indicating the absence of renal damage.
X-rays of the right foot were taken as shown in Figure 3, 4. The possibility of DVT (deep vein thrombosis) was ruled out by way of an ultrasound Doppler.
Blood tests were also conducted for underlying causes, HBV (Hepatitis B), HCV (Hepatitis C) and HIV (Human Immunodeficiency virus) were all negative.
Treatment
The patient was admitted for observation under orthopedic care and started on IV antibiotics (Co-amoxiclav and amikacin), along with supportive treatment and daily dressing. He was also recommended icing and elevation of his foot.
Two days later, the patient was recommended to undergo a wound debridement procedure (Figure 5).
Wound debridement was conducted under general anesthesia with a singular incision being placed in the medial aspect at the junction of the medial and dorsal border of the foot. There was full-thickness skin necrosis along with scant pus discharge, the necrotic tissue was removed, and the wound was washed using saline, hydrogen peroxide and pyodine. Tissue samples were taken during the debridement procedure. These tissue samples were sent for culture and sensitivity studies.
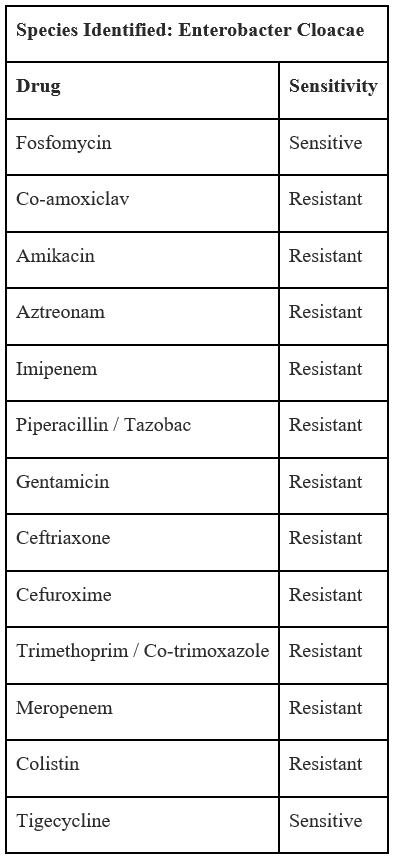
On the seventh postoperative day, a relook debridement was conducted to remove further necrosed tissue, it was conducted under general anaesthesia. Culture and sensitivity reports showed that the wound infection was caused by Enterobacter Cloacae resistant to most antibiotics, susceptible only to Fosfomycin and Tigecycline as seen in Table 2. The patient was thus started on Fosfomycin treatment.
After two days of Fosfomycin therapy, the patient had significant improvement in wound healing. Despite discussing possible future complications and the doctor's recommendation, the patient requested leave from the hospital and was allowed to LAMA (Leave against medical advice).
The patient agreed to return for follow-up visits and their progress was followed over the next two months on an outpatient basis, until complete recovery. The patient was also recommended physiotherapy treatment for the improvement of functional mobility.
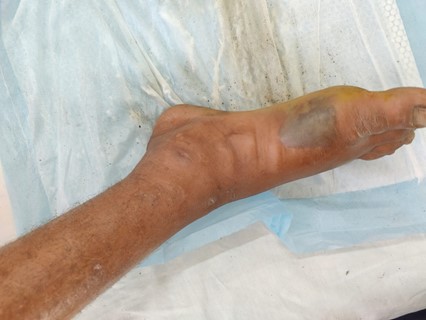
Figure 1: Wound at initial presentation.
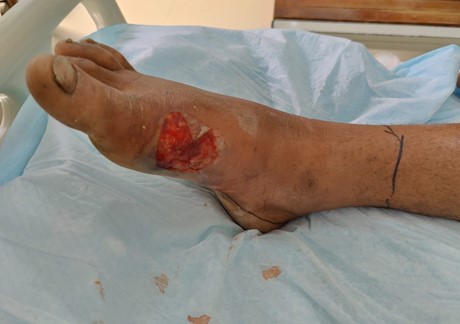
Figure 2: Wound after rupturing the blister.
Table 1: Patients baseline investigations.

Figure 3: X-ray right foot, AP view.
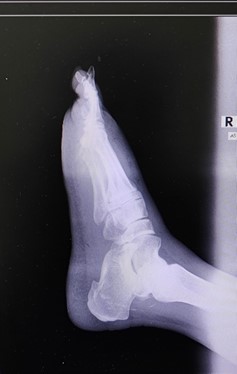
Figure 4: X-ray right foot, lateral view.
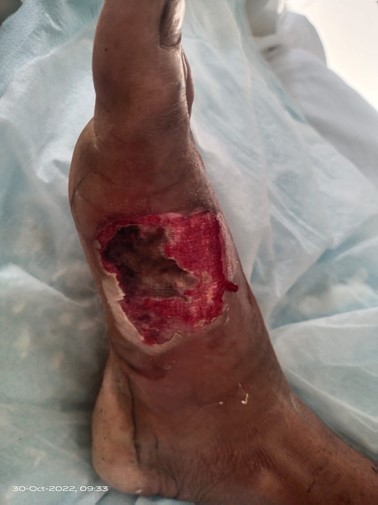
Figure 5: Wound post-debridement.
Table 2: Tissue culture and sensitivity report.
Discussion
Widespread incidence and detection of CREC pose a massive threat to public health. It is therefore of the highest importance to characterize and classify the clinical and molecular features of these strains. This will allow for further study into the modes of development of such resistant strains and further lead to the development of preventative methods.
In a study, since the first identification of CREC strains, in 2012, there has been a rapid increase in the incidence, having increased to 8.11% in 2018 and 6.48% in 2019 [9]. This further indicates its importance as a rapidly emerging nosocomial infection.
CREC has been established as the 3rd most common carbapenem-resistant Enterobacteriaceae in China [10].
The emergence of these drug-resistant strains further means that treatment options for these infections are limited, making them an even greater threat. It was seen that combination treatment with multiple antibiotics, like meropenem, polymyxin B, tigecycline and amikacin showed significant positive coactivity [11,12]. However, it is important to evaluate the optimal treatment for each strain based on sequence types and resistant genotypes.
Possible determinants for the increase in the emergence of these carbapenem-resistant ECC strains can be 1) the widespread use of broad-spectrum antibiotics (including penicillins, first and second-generation cephalosporins, and carbapenems), 2) the use of invasive devices (mechanical ventilation, nasogastric and feeding tubes, urinary Foley's catheterization, central venous catheter, and the use of parenteral nutrition), 3) surgical procedures (associated with prolonged hospitalization and increased risk of acquisition of these drug-resistant strains) [13]. It can also be due to the enhanced ability of ECC organisms to acquire drug-resistance genes, thus leading to a rapid increase in CREC cases [14].
Previous studies conducted focus on the prevalence of CREC as a nosocomial infection. However, there is a significant threat of these strains spreading to the community. This patient had no history of prior hospitalization, no comorbidities and no recent history of antibiotic use. The patient had therefore acquired this infection from within the community. There is, therefore, an immediate need to take effective measures to prevent the spread of such infections into the community and to further treat and control this rapidly growing threat.
Conclusion
With the rapid emergence of CREC strains, it is imperative that immediate action be taken to investigate and prevent their further development. CREC, while previously found in hospital-based settings has now emerged in community-acquired settings, which is a major warning for the medical community and can pose a massive public health risk. Prevention of the strains and containment of the already developed strains must be achieved if we are to prevent this growing infectious catastrophe.
Disclosures
Human subjects: Consent was obtained by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Sanders WE, Sanders CC. Enterobacter spp.: Pathogens poised to flourish at the turn of the century. Clinical Microbiology Reviews, 1997; 10(2): 220–241.
- Davin-Regli A, Lavigne J-P, Pagès J-M. enterobacter: Update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clinical Microbiology Reviews, 2019; 32(4).
- Mezzatesta ML, Gona F, Stefani S. enterobacter cloacae complex: Clinical impact and emerging antibiotic resistance. Future Microbiology, 2012; 7(7): 887–902.
- Seeberg AH, Tolxdorff-Neutzling RM, Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrobial Agents and Chemotherapy, 1983; 23(6): 918–925.
- Naas T, Nordmann P. Analysis of a carbapenem-hydrolyzing class A beta-lactamase from Enterobacter cloacae and of its lysr-type regulatory protein. Proceedings of the National Academy of Sciences, 1994; 91(16): 7693–7697.
- Tarumoto N, Kodana M, Watanabe N, Sakai J, Imai K, Yamaguchi T, et al. First report of the isolation of blaimi-1-producing colistin-heteroresistant Enterobacter cloacae in Japan, September 2016. Journal of Infection and Chemotherapy, 2018; 24(11): 941–943.
- Uechi K, Tada T, Shimada K, Nakasone I, Kirikae T, Fujita J. Emergence of a carbapenem-resistant and colistin-heteroresistant Enterobacter cloacae clinical isolate in Japan. Journal of Infection and Chemotherapy, 2019; 25(4): 285–288.
- Chavda B, Lv J, Hou M, Chavda KD, Kreiswirth BN, Feng Y, et al. Coidentification of mcr-4.3 and bla ndm-1 in Clinical Enterobacter cloacae isolate from China. Antimicrobial Agents and Chemotherapy, 2018; 62(10).
- Chen J, Tian S, Nian H, Wang R, Li F, Jiang N, et al. Carbapenem-resistant Enterobacter cloacae complex in a tertiary hospital in Northeast China, 2021; 2010-2019.
- Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: Report from the China Cre Network. Antimicrobial Agents and Chemotherapy, 2018; 62(2).
- Zhao Y, Li C, Zhang J, Fu Y, Hu K, Su S, et al. The in vitro activity of polymyxin B and Tigecycline alone and combination with other antibiotics against carbapenem-resistant Enterobacter cloacae complex isolates, including high-risk clones. Annals of Translational Medicine, 2019; 7(23): 779.
- Alves PH, Boff RT, Barth AL, Martins AF. Synergy of Polymyxin B, Tigecycline and meropenem against carbapenem-resistant Enterobacter cloacae complex isolates. Diagnostic Microbiology and Infectious Disease, 2019; 94(1): 81–85.
- Tian X, Huang C, Ye X, Jiang H, Zhang R, Hu X, et al. carbapenem-resistant enterobacter cloacae causing nosocomial infections in southwestern China: Molecular epidemiology, risk factors, and predictors of mortality. Infection and Drug Resistance, 2020; 13: 129–137.
- Gomez-Simmonds A, Annavajhala MK, Wang Z, Macesic N, Hu Y, Giddins MJ, et al. Genomic and geographic context for the evolution of high-risk carbapenem-resistant enterobacter cloacae complex clones ST171 and ST78. mBio, 2018; 9(3).

